Company: Medisky Pharmaceuticals Pvt. Ltd.
Industry: Pharmaceutical Manufacturing
Job Location: Talod, Sabarkantha & Ahmedabad, Gujarat
Experience: 1 to 5 Years
Key Skills: Regulatory Affairs, ACTD Dossier, eCTD Dossier, International Marketing, Pharma Export, LATAM, SEA, Trade Fairs
Are you a skilled pharmaceutical professional looking to accelerate your career with a WHO-GMP certified manufacturer? Medisky Pharmaceuticals Pvt. Ltd., a leading name in pharma manufacturing, is expanding its team and looking for dynamic individuals to join our growing family.
About Medisky Pharmaceuticals Pvt. Ltd.
At Medisky Pharmaceuticals, we are dedicated to manufacturing high-quality pharmaceutical products that meet global standards. Our diverse portfolio includes General Tablets, Capsules, Ointments, Gels, Creams, Lotions, and Shampoos.
Our state-of-the-art manufacturing plant is located in Talod, Sabarkantha, Gujarat, and is certified under the stringent WHO-GMP (World Health Organization – Good Manufacturing Practices) guidelines. We also hold other international accreditations, underscoring our commitment to quality, safety, and excellence in every product we produce. Our mission is to deliver effective healthcare solutions both in India and across the globe.
Open Positions and Detailed Job Description
We are currently seeking talented candidates for the following roles:
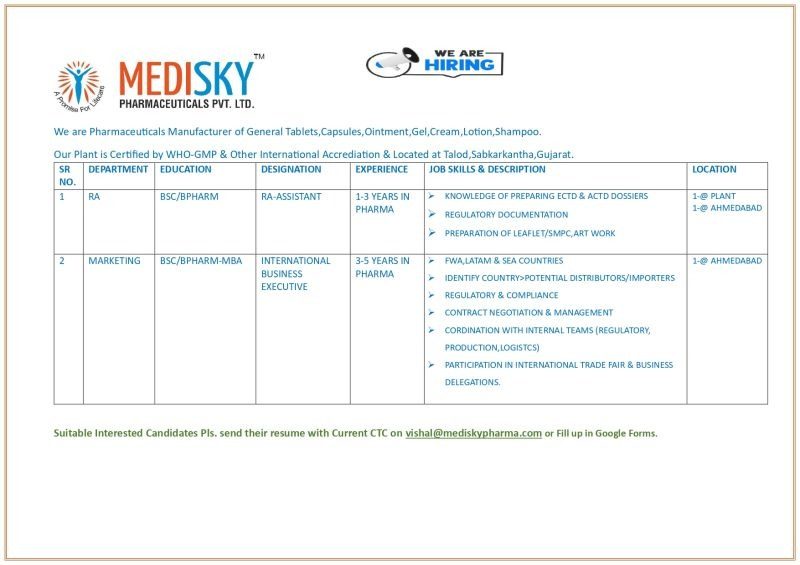
1. Position: Regulatory Affairs (RA) Assistant
- Department: Regulatory Affairs (RA)
- Designation: RA-Assistant
- Location: 1 Opening at Plant (Talod, Sabarkantha), 1 Opening in Ahmedabad
- Education Required: B.Sc. or B.Pharm
- Experience Required: 1 to 3 years in the pharmaceutical industry.
Key Job Skills & Description:
The ideal candidate will have proven experience in pharma regulatory affairs. Your primary responsibilities will include:
- Regulatory Documentation: Preparing, reviewing, and compiling various regulatory submissions.
- Dossier Preparation: In-depth knowledge of preparing eCTD and ACTD dossiers for drug approvals.
- Leaflet & Artwork: Preparation and review of product leaflets, SMPC (Summary of Product Characteristics), and managing artwork approvals.
- Compliance: Ensuring all documentation complies with national and international regulatory standards.
This is an excellent opportunity for those who want to deepen their expertise in regulatory affairs and work with a certified WHO-GMP plant.
2. Position: International Business Executive
- Department: Marketing
- Designation: International Business Executive
- Location: Ahmedabad
- Education Required: B.Sc./B.Pharm with an MBA
- Experience Required: 3 to 5 years in pharmaceutical marketing or exports.
Key Job Skills & Description:
We are looking for a proactive professional to drive our international business growth. Your role will be crucial in expanding our footprint in key global markets.
- Market Expansion: Focus on FWA (Free Sale Certificate, Wholesale, Advertising) requirements for LATAM (Latin America) and SEA (South East Asia) countries.
- Business Development: Identify, evaluate, and onboard potential distributors and importers in target international markets.
- Contract Management: Lead contract negotiation and management with international partners.
- Regulatory Coordination: Work closely with internal regulatory and compliance teams to ensure market entry requirements are met.
- Internal Coordination: seamless coordination with production and logistics teams to ensure timely order fulfillment.
- Global Representation: Represent the company at international trade fairs and business delegations.
This role is perfect for someone with a strong understanding of pharma export procedures and a passion for building global business relationships.
How to Apply
Suitable and interested candidates are requested to apply at the earliest.
Please send your detailed resume/CV, mentioning your Current CTC (Cost to Company), to our HR team at:
Email: vishal@mediskypharma.com