Micro Labs Limited is a renowned multinational pharmaceutical company headquartered in Bangalore, India, with a strong presence in Goa at its state-of-the-art manufacturing facility in Verna Industrial Estate. Established in 1973, Micro Labs specializes in the development, manufacturing, and marketing of generic drugs, formulations, and active pharmaceutical ingredients (APIs). The company exports to over 50 countries and is known for its commitment to quality, innovation, and regulatory compliance, including USFDA, WHO, and GMP standards.
With a workforce of over 7,000 employees, Micro Labs emphasizes employee growth, cutting-edge R&D, and sustainable practices. The Verna, Goa plant is a hub for pharma production in India, focusing on tablets, capsules, injectables, and ophthalmics. Joining Micro Labs means contributing to global healthcare while advancing your career in a dynamic environment. For more on Micro Labs careers, visit their official site or follow updates on Pharmabharat.org, India’s top platform for pharma job vacancies.
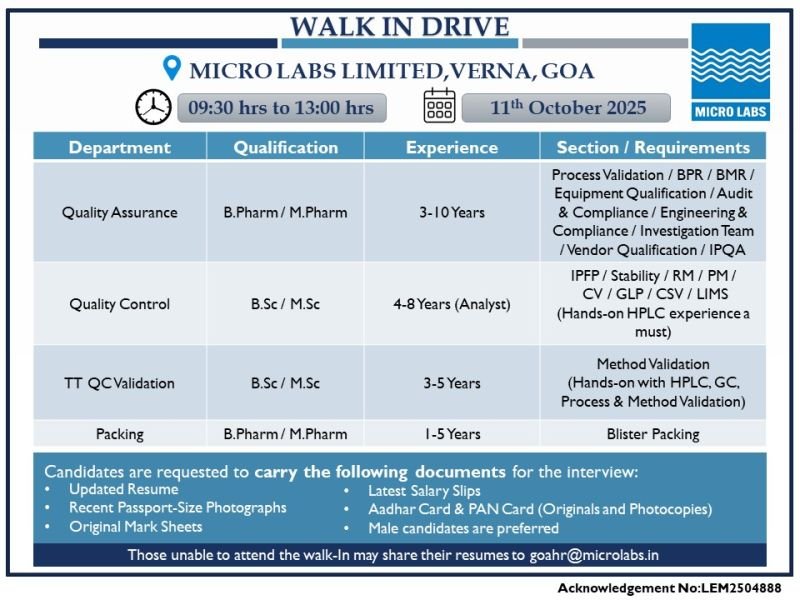
Job Descriptions: Exciting Pharma Opportunities at Micro Labs Walk-in Drive
Micro Labs is recruiting for multiple roles in Quality Assurance, Quality Control, TT QC Validation, and Packing at their Verna, Goa facility. These pharma jobs in Goa require hands-on experience in critical areas like HPLC, GC, validation, and compliance. Below are the detailed job descriptions for Micro Labs 2025:
1. Quality Assurance (QA) Positions
- Department: Quality Assurance
- Qualification: B.Pharm / M.Pharm
- Experience: 3-10 Years
- Key Responsibilities:
- Handle process validation, Batch Production Record (BPR), and Batch Manufacturing Record (BMR) reviews.
- Conduct equipment qualification, internal audits, and compliance checks.
- Manage engineering and compliance activities, lead investigation teams, perform vendor qualification, and oversee In-Process Quality Assurance (IPQA).
- Skills Required: Strong knowledge of GMP, regulatory audits, and documentation. Ideal for professionals seeking Quality Assurance jobs in pharma.
- Why Apply?: Play a pivotal role in ensuring product safety and regulatory adherence in a high-volume manufacturing setup.
2. Quality Control (QC) Analyst Roles
- Department: Quality Control
- Qualification: B.Sc / M.Sc (Chemistry or related fields)
- Experience: 4-8 Years (as Analyst)
- Key Responsibilities:
- Perform testing in IPFP (In-Process Finished Product), stability studies, Raw Material (RM), Packaging Material (PM), Chemical Validation (CV), Good Laboratory Practice (GLP), Computer System Validation (CSV), and Laboratory Information Management System (LIMS).
- Hands-on operation of HPLC (High-Performance Liquid Chromatography) for analytical testing – mandatory experience required.
- Skills Required: Proficiency in instrumental analysis, wet chemistry, and data integrity. Perfect for Quality Control jobs in pharma with a focus on analytical techniques.
- Why Apply?: Opportunity to work in a GLP-compliant lab, contributing to product quality from raw materials to final release.
3. TT QC Validation Positions
- Department: TT QC Validation
- Qualification: B.Sc / M.Sc
- Experience: 3-5 Years
- Key Responsibilities:
- Execute method validation protocols using HPLC, GC (Gas Chromatography), and other instruments.
- Conduct process validation and method validation in pharma, ensuring accuracy and reproducibility.
- Skills Required: Expertise in validation methodologies, ICH guidelines, and troubleshooting analytical equipment. Essential for pharma validation jobs.
- Why Apply?: Advance your expertise in cutting-edge validation techniques at a facility compliant with international standards.
4. Packing Roles
- Department: Packing
- Qualification: B.Pharm / M.Pharm
- Experience: 1-5 Years
- Key Responsibilities:
- Oversee blister packing operations, including line setup, quality checks, and efficiency improvements.
- Ensure compliance with packing standards and documentation.
- Skills Required: Knowledge of packaging machinery, GMP for secondary packaging, and defect analysis. Suited for entry-to-mid-level pharma packing jobs.
- Why Apply?: Hands-on role in the final stages of drug production, with growth potential in operations.
These Micro Labs job openings emphasize practical skills like HPLC experience in pharma, audit compliance, and validation, making them ideal for mid-career professionals in the pharmaceutical sector.
Eligibility Criteria for Micro Labs Walk-in Interview 2025
- Educational Qualifications: B.Pharm, M.Pharm, B.Sc, or M.Sc in relevant fields (Pharmacy, Chemistry, or Life Sciences).
- Experience Level: 1-10 Years, depending on the role (e.g., 4-8 years for QC Analysts with mandatory hands-on HPLC experience).
- Preferences: Male candidates preferred. Candidates must have a strong background in pharma quality control, validation, or packing.
- Location: Verna, Goa – Relocation assistance may be available for eligible candidates.
- Acknowledgement No.: LEM2504888 (Reference this in applications).
Pharmabharat.org recommends checking your eligibility against these pharma jobs in Goa criteria to maximize your chances.
How to Apply: Walk-in Drive Details and Application Process
Micro Labs is conducting a walk-in interview in Goa on Saturday, 11th October 2025, from 09:30 hrs to 13:00 hrs at their facility:
Micro Labs Limited, Verna Industrial Estate, Goa.
(Contact: 000 000 for venue confirmation – limited spots available!)
Documents to Carry for the Walk-in:
- Updated Resume (highlighting HPLC experience, validation projects, and pharma compliance).
- Recent Passport-Size Photographs (2-3 nos.).
- Original Mark Sheets and Certificates (from 10th onwards).
- Latest Salary Slips (last 3 months).
- Aadhar Card & PAN Card (Originals and Photocopies).