Ciron Drugs is a well-established pharmaceutical company with a strong reputation for quality and innovation. Operating multiple, specialized manufacturing units, the company is a significant player in the production of various drug formulations. The two plants relevant to this drive are:
- Boisar Plant: A dedicated Injectable Plant, focusing on sterile and precision-based manufacturing.
- Palghar Plant: A robust Tablet and Capsule Formulation Plant, handling solid oral dosages.
By joining Ciron Drugs, you become part of an organization that values quality, invests in its people, and contributes significantly to the healthcare sector.
Job Description: Quality Control (QC) Opportunities
We are looking for enthusiastic and detail-oriented individuals for our Quality Control (QC) department across various sections. This is an urgent requirement with multiple vacancies for both freshers and experienced candidates.
Walk-In Drive Details:
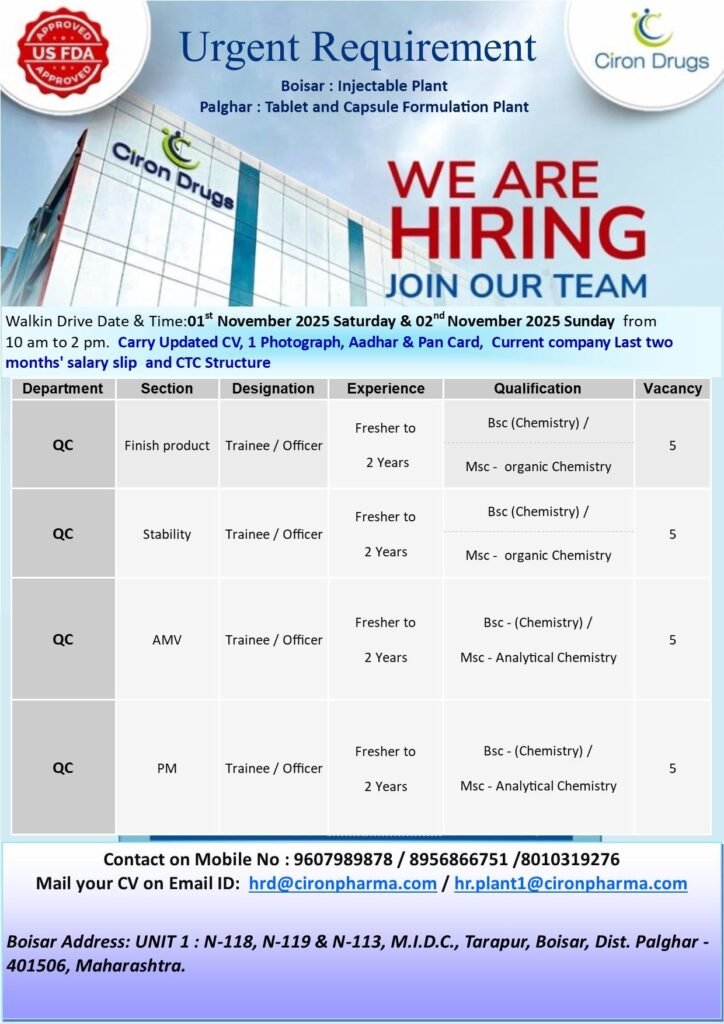
- Date: Saturday, 1st November 2025 & Sunday, 2nd November 2025
- Time: 10:00 AM to 2:00 PM
Venue for the Interview:
Ciron Drugs – UNIT 1
N-118, N-119 & N-113, M.I.D.C., Tarapur,
Boisar, Dist. Palghar – 401506, Maharashtra.
Detailed Job Openings
Here is a breakdown of the current vacancies in the QC department:
| Department | Section | Designation | Experience Required | Qualification Required | Vacancies |
|---|---|---|---|---|---|
| QC | Finish Product | Trainee / Officer | Fresher to 2 Years | BSc (Chemistry) / MSc Organic Chemistry | 5 |
| QC | Stability | Trainee / Officer | Fresher to 2 Years | BSc (Chemistry) / MSc Organic Chemistry | 5 |
| QC | AMV | Trainee / Officer | Fresher to 2 Years | BSc (Chemistry) / MSc Analytical Chemistry | 5 |
| QC | PM | Trainee / Officer | Fresher to 2 Years | BSc (Chemistry) / MSc Analytical Chemistry | 5 |
Key Responsibilities (May Vary by Section):
- Performing chemical and instrumental analysis of raw materials, in-process, and finished products.
- Conducting stability studies as per ICH guidelines.
- Handling sophisticated instruments like HPLC, GC, UV-Vis Spectrophotometer, etc. (for relevant roles).
- Ensuring all documentation is maintained as per cGMP and SOP guidelines.
- Participating in method validation and verification activities.
How to Apply for the Walk-In
Applying is straightforward. Follow these steps to ensure your participation:
- Prepare Your Documents: Candidates must carry the following documents to the walk-in venue:
- Updated CV/Resume
- 1 Recent Photograph
- Aadhar Card & PAN Card (Photocopies and Originals for verification)
- Last two months’ salary slips (if experienced)
- Current CTC Structure (if experienced)
- Attend the Drive: Walk in directly to the provided Boisar address on 1st or 2nd November 2025 between 10:00 AM and 2:00 PM. Dress professionally and be prepared for a potential on-the-spot interview.
Prefer to Apply Online?
You can also pre-emptively send your CV to the HR department to express your interest.
- Contact Mobile Numbers: 9607989878 / 8956866751 / 8010319276
- Email Your CV to: hrd@cironpharma.com / hr.plant1@cironpharma.com