Alembic Pharmaceuticals Limited is one of India’s leading, integrated pharmaceutical companies. With a strong legacy and a focus on innovation, Alembic has a significant presence in the manufacturing of Formulations (OSD & Injectable) and Active Pharmaceutical Ingredients (API). The company is renowned for its commitment to quality, compliance with global cGMP standards, and its vibrant work culture, officially recognized as a Great Place to Work. A career at Alembic means growth, learning, and contributing to a company that impacts global healthcare.
Job Openings & Detailed Job Description
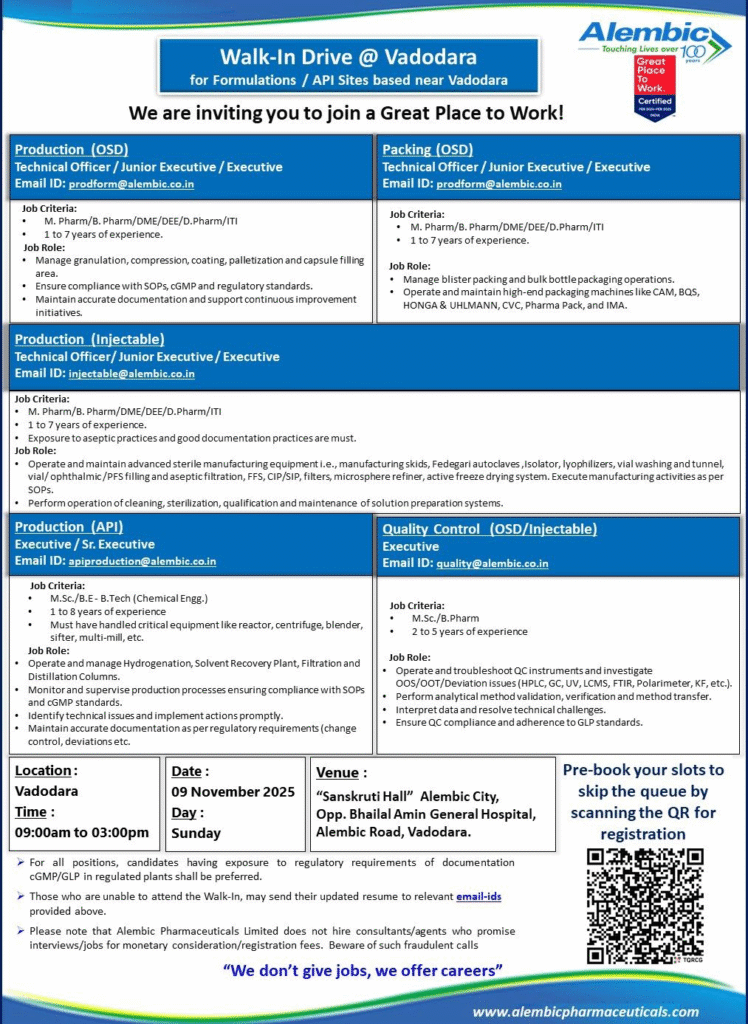
Alembic is hiring for multiple departments. Here is the complete breakdown of the vacancies:
1. Production (OSD)
- Post: Technical Officer / Junior Executive / Executive
- Email ID: prodform@alembic.co.in
- Job Description:
- Manage key processes in solid dosage manufacturing: granulation, compression, coating, palletization, and capsule filling.
- Ensure strict compliance with SOPs, CGMP, and regulatory standards.
- Maintain accurate documentation and support continuous improvement projects.
2. Packing (OSD)
- Post: Technical Officer / Junior Executive / Executive
- Email ID: prodform@alembic.co.in
- Job Description:
- Manage blister packing and bulk bottle packaging operations.
- Operate and maintain high-end packaging machines like CAM, BQS, HONGA & UHLMANN, CVC, Pharma Pack, and IMA.
3. Production (Injectable)
- Post: Technical Officer / Junior Executive / Executive
- Email ID: injectable@alembic.co.in
- Job Description:
- Operate and maintain advanced sterile manufacturing equipment including autoclaves, isolators, lyophilizers, and filling machines.
- Execute manufacturing activities as per SOPs with a strong focus on aseptic practices.
- Perform cleaning, sterilization, and qualification of systems.
4. Production (API)
- Post: Executive / Sr. Executive
- Email ID: apiproduction@alembic.co.in
- Job Description:
- Operate and manage critical API plant equipment like reactors, hydrogenation units, centrifuge, and solvent recovery plants.
- Monitor production processes, ensuring compliance with SOPs and CGMP.
- Identify technical issues and implement corrective actions promptly.
- Maintain documentation for change control and deviations.
5. Quality Control (OSD/Injectable)
- Post: Executive
- Email ID: quality@alembic.co.in
- Job Description:
- Operate and troubleshoot sophisticated QC instruments (HPLC, GC, UV, LCMS, etc.).
- Investigate OOS/OOT/Deviation issues.
- Perform analytical method validation, verification, and transfer.
- Ensure compliance with GLP standards.
Eligibility Criteria (Job Criteria)
- For Production & Packing (OSD/Injectable): M.Pharm / B.Pharm / DME / DEE / D.Pharm / ITI with 1 to 7 years of experience.
- For Production (API): M.Sc. / B.E./B.Tech (Chemical Engineering) with 1 to 8 years of experience. Must have handled reactors, centrifuges, etc.
- For Quality Control: M.Sc. / B.Pharm with 2 to 5 years of experience.
- Preferred Skill: Candidates with exposure to regulatory documentation, cGMP, and GLP in regulated plants will be preferred.
Walk-In Interview Details
- Date: 09 November 2025 (Sunday)
- Time: 9:00 AM to 3:00 PM
- Venue: “Sanskruti Hall”, Alembic City, Opp. Bhailal Amin General Hospital, Alembic Road, Vadodara.
How to Apply
There are two ways to apply for these exciting career opportunities at Alembic Pharmaceuticals:
- Attend the Walk-In Drive: Carry your updated resume, relevant documents, and walk in directly to the venue on the specified date and time.
- Pro Tip: Pre-book your slots to skip the queue by scanning the QR code provided in the original advertisement for registration.
- Email Your Resume: If you are unable to attend the walk-in, you can send your updated resume to the relevant email IDs provided for each department above.