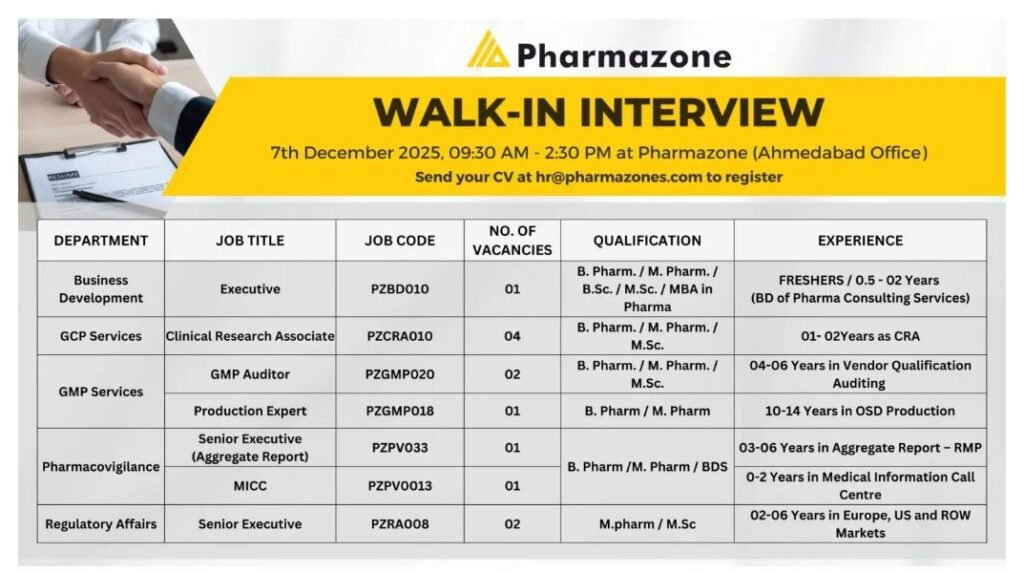
Are you passionate about advancing healthcare through pharmaceutical innovation? Pharmazone, a leading player in pharma consulting and services, is hosting a walk-in interview event in Ahmedabad on December 7, 2025, from 9:30 AM to 2:30 PM. This is your chance to join a dynamic team in roles spanning business development, clinical research, GMP services, pharmacovigilance, and regulatory affairs. Whether you’re a fresher or an experienced professional in the pharma industry, these positions offer growth in a thriving sector. Don’t miss out on these pharma jobs in Ahmedabad—register your CV today and step into a rewarding career in pharmaceuticals.
Key Responsibilities
Pharmazone is seeking talented individuals for various roles, each with specific duties tailored to drive excellence in pharma operations:
- Business Development Executive (PZBD010): Focus on expanding pharma consulting services, identifying new clients, and building partnerships in the pharmaceutical market.
- Clinical Research Associate (PZCRA010): Oversee clinical trials, ensure compliance with GCP guidelines, and manage data for drug development projects.
- GMP Auditor (PZGMP020): Conduct vendor qualification audits, maintain GMP standards, and ensure quality in pharmaceutical production.
- Production Expert (PZGMP018): Lead OSD (Oral Solid Dosage) production with 10-14 years of experience, optimizing manufacturing processes.
- Senior Executive (Aggregate Report) (PZPV033): Prepare aggregate reports and risk management plans for pharmacovigilance, ensuring regulatory compliance.
- Senior Executive (Pharmacovigilance) (PZPV0013): Handle medical information call center operations, process adverse event reports, and support drug safety initiatives.
- Regulatory Affairs Executives (PZRA008): Manage submissions for Europe, US, and ROW markets, ensuring adherence to international regulatory standards.
These pharma jobs in Ahmedabad emphasize collaboration, innovation, and adherence to global pharma standards, making them ideal for professionals committed to improving patient outcomes.
Qualifications and Experience
To qualify for these pharma career opportunities, candidates should meet the following criteria:
- Business Development Executive: B. Pharm., M. Pharm., B.Sc., M.Sc., or MBA in Pharma; Freshers or 0.5-2 years in pharma consulting services.
- Clinical Research Associate: B. Pharm. or M. Pharm.; 1-2 years as CRA in clinical research.
- GMP Auditor: B. Pharm. or M. Pharm.; 4-6 years in vendor qualification auditing.
- Production Expert: B. Pharm. or M. Pharm.; 10-14 years in OSD production.
- Senior Executive (Aggregate Report): B. Pharm., M. Pharm., or BDS; 3-6 years in aggregate report and RMP preparation.
- Senior Executive (Pharmacovigilance): M. Pharm. or M.Sc.; 0-2 years in medical information call center (MICC).
- Regulatory Affairs Executives: B. Pharm. or M. Pharm.; 2-6 years in regulatory affairs for Europe, US, and ROW markets.
Strong communication skills, attention to detail, and knowledge of pharma regulations are essential for all roles.
Benefits of Joining Pharmazone
Working at Pharmazone offers more than just a job—it’s a pathway to professional growth in the pharma industry. Enjoy competitive salaries, health benefits, opportunities for skill development through training programs, and a supportive work environment. As part of a leading pharma company, you’ll contribute to life-saving innovations while advancing your career in pharma jobs in India.
How to Apply
Ready to apply for these pharma jobs in Ahmedabad? Send your updated CV to hr@pharmazones.com to register for the walk-in interview. The event is scheduled for December 7, 2025, from 9:30 AM to 2:30 PM at Pharmazone’s Ahmedabad office. Prepare to bring your resume, portfolio, and enthusiasm for an on-the-spot interview. Early registration is recommended to secure your spot