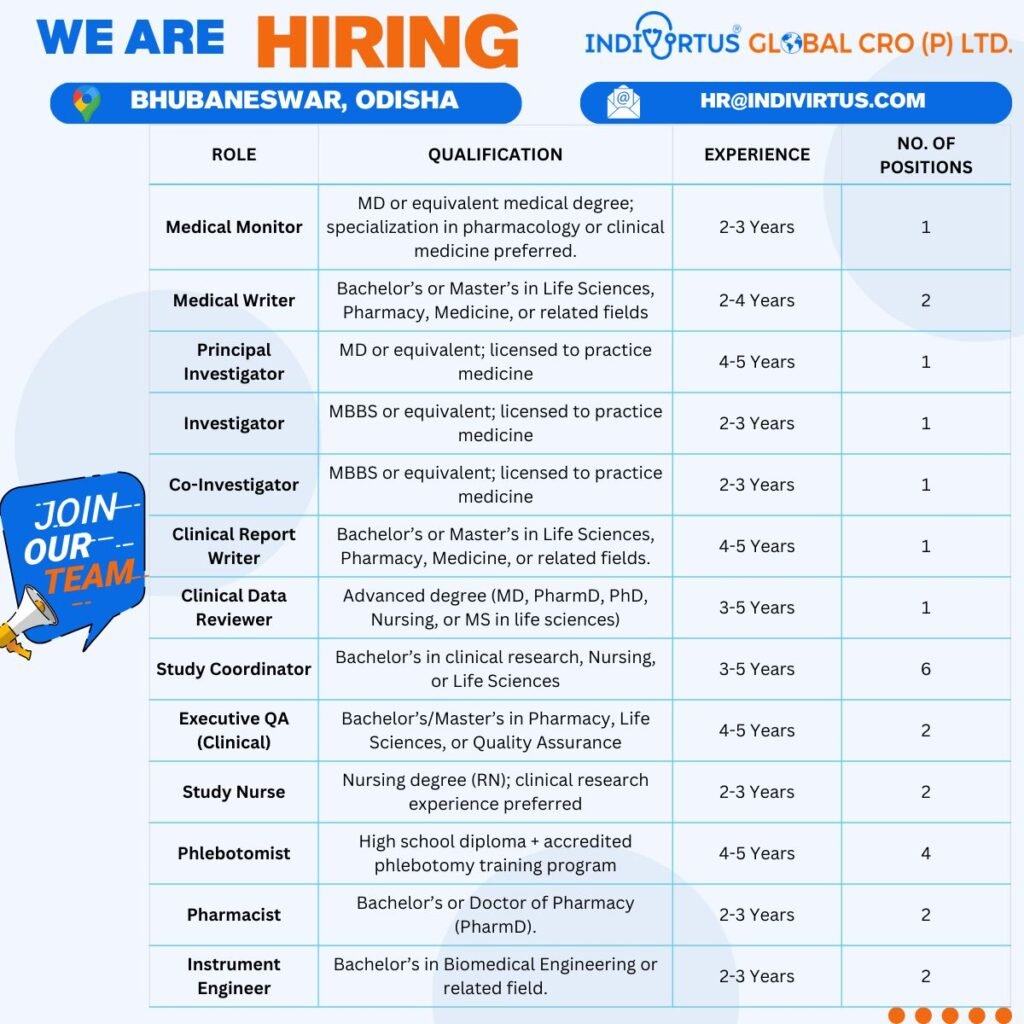
Are you passionate about advancing clinical trials and pharmaceutical research? INDIVORTUS GLOBAL CRO (P) LTD., a leading contract research organization in Bhubaneswar, Odisha, is expanding its team and seeking talented professionals for various clinical research jobs. With a focus on innovative drug development and ethical practices, we offer a dynamic work environment where you can contribute to life-saving medical breakthroughs. Whether you’re a seasoned medical professional or a fresh graduate in life sciences, these CRO jobs in India provide excellent career growth in the booming pharma sector. Explore our current openings and apply today to join a team dedicated to excellence in clinical research.
Medical Monitor
Responsibilities: Oversee clinical trial protocols, ensure patient safety, review adverse events, and collaborate with investigators to maintain trial integrity in pharma CRO settings.
Qualifications: MD or equivalent medical degree; specialization in pharmacology or clinical medicine preferred. 2-3 years of experience.
Benefits: Competitive salary (INR 80,000-120,000/month), health insurance, professional development opportunities, and work-life balance in a supportive clinical research environment.
Application Instructions: Send your resume and cover letter to HR@INDIVIRTUS.COM with the subject “Medical Monitor Application.”
Medical Writer
Responsibilities: Develop and edit clinical study documents, regulatory submissions, and reports for pharmaceutical trials, ensuring accuracy and compliance with industry standards.
Qualifications: Bachelor’s or Master’s in Life Sciences, Pharmacy, Medicine, or related fields. 2-4 years of experience.
Benefits: Attractive compensation (INR 50,000-80,000/month), flexible working hours, skill-building workshops, and career advancement in clinical research jobs.
Application Instructions: Email your CV to HR@INDIVIRTUS.COM with “Medical Writer Position” in the subject line.
Principal Investigator
Responsibilities: Lead clinical trials, manage study teams, ensure regulatory compliance, and oversee patient recruitment and data collection at research sites.
Qualifications: MD or equivalent; licensed to practice medicine. 4-5 years of experience.
Benefits: High earning potential (INR 100,000-150,000/month), comprehensive benefits package including retirement plans, and opportunities for leadership roles in CRO jobs Bhubaneswar.
Application Instructions: Apply by sending your application to HR@INDIVIRTUS.COM, subject: “Principal Investigator Role.”
Investigator
Responsibilities: Conduct clinical assessments, monitor patient progress, and ensure adherence to trial protocols in pharmaceutical research.
Qualifications: MBBS or equivalent; licensed to practice medicine. 2-3 years of experience.
Benefits: Competitive pay (INR 70,000-100,000/month), medical coverage, paid leave, and professional networking in clinical research.
Application Instructions: Submit your resume to HR@INDIVIRTUS.COM with “Investigator Application” as the subject.
Co-Investigator
Responsibilities: Assist in trial execution, data analysis, and patient care coordination under the Principal Investigator’s guidance.
Qualifications: MBBS or equivalent; licensed to practice medicine. 2-3 years of experience.
Benefits: Rewarding salary (INR 60,000-90,000/month), team collaboration perks, and growth prospects in pharma CRO careers.
Application Instructions: Email HR@INDIVIRTUS.COM with your details, subject: “Co-Investigator Position.”
Clinical Report Writer
Responsibilities: Compile and analyze clinical data, write detailed reports, and support regulatory filings for drug development studies.
Qualifications: Bachelor’s or Master’s in Life Sciences, Pharmacy, Medicine, or related fields. 4-5 years of experience.
Benefits: Strong compensation (INR 60,000-90,000/month), remote work options, and continuous learning in clinical research jobs India.
Application Instructions: Apply via email to HR@INDIVIRTUS.COM, subject: “Clinical Report Writer.”
Clinical Data Reviewer
Responsibilities: Review and validate clinical trial data, ensure data integrity, and prepare summaries for regulatory submissions.
Qualifications: Advanced degree (MD, PharmD, PhD, Nursing, or MS in life sciences). 3-5 years of experience.
Benefits: Competitive package (INR 70,000-110,000/month), health benefits, and career progression in CRO jobs.
Application Instructions: Send application to HR@INDIVIRTUS.COM with “Clinical Data Reviewer” in the subject.
Study Coordinator
Responsibilities: Coordinate study logistics, manage documentation, liaise with sites, and ensure smooth trial operations.
Qualifications: Bachelor’s in clinical research, Nursing, or Life Sciences. 3-5 years of experience.
Benefits: Attractive salary (INR 40,000-70,000/month), team incentives, and flexible schedules in pharma research.
Application Instructions: Email HR@INDIVIRTUS.COM, subject: “Study Coordinator Application.”
Executive QA (Clinical)
Responsibilities: Conduct quality audits, ensure compliance with GCP and regulatory standards, and improve clinical processes.
Qualifications: Bachelor’s/Master’s in Pharmacy, Life Sciences, or Quality Assurance. 4-5 years of experience.
Benefits: Good pay (INR 60,000-90,000/month), professional certifications, and stability in clinical research careers.
Application Instructions: Apply to HR@INDIVIRTUS.COM with “Executive QA Role.”
Study Nurse
Responsibilities: Provide nursing care to trial participants, administer treatments, and collect vital data in clinical settings.
Qualifications: Nursing degree (RN); clinical research experience preferred. 2-3 years of experience.
Benefits: Competitive earnings (INR 40,000-60,000/month), shift allowances, and healthcare perks.
Application Instructions: Send resume to HR@INDIVIRTUS.COM, subject: “Study Nurse Position.”
Phlebotomist
Responsibilities: Perform blood draws, sample collection, and ensure proper labeling for clinical trials.
Qualifications: High school diploma + accredited phlebotomy training program. 4-5 years of experience.
Benefits: Decent salary (INR 25,000-40,000/month), overtime pay, and job security in pharma CRO.
Application Instructions: Email HR@INDIVIRTUS.COM with “Phlebotomist Application.”
Pharmacist
Responsibilities: Manage drug dispensing, ensure medication safety, and support trial protocols in pharmaceutical research.
Qualifications: Bachelor’s or Doctor of Pharmacy (PharmD). 2-3 years of experience.
Benefits: Attractive package (INR 50,000-80,000/month), licensing support, and growth in clinical jobs.
Application Instructions: Apply via email to HR@INDIVIRTUS.COM, subject: “Pharmacist Role.”
Instrument Engineer
Responsibilities: Maintain and calibrate medical equipment, troubleshoot issues, and ensure compliance in clinical labs.
Qualifications: Bachelor’s in Biomedical Engineering or related field. 2-3 years of experience.
Benefits: Competitive pay (INR 50,000-80,000/month), technical training, and innovative work environment.
Application Instructions: Send application to HR@INDIVIRTUS.COM with “Instrument Engineer.”