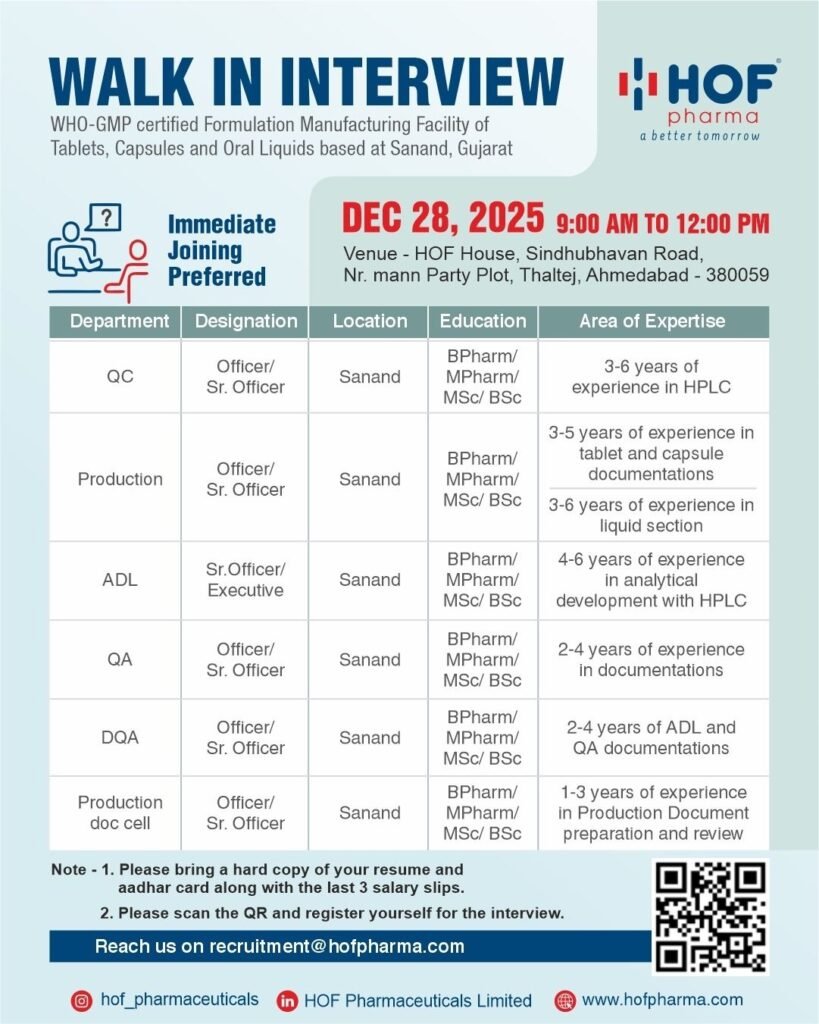
HOF Pharmaceuticals, a WHO-GMP certified formulation manufacturing facility specializing in tablets, capsules, and oral liquids, is conducting a walk-in interview for multiple positions in QC, QA, Production, Analytical Development (ADL), and Document Control at Sanand, Gujarat. Immediate joining is preferred.
Interview Details:
- Date: December 28, 2025
- Time: 9:00 AM – 12:00 PM
- Venue: HOF House, Sindhubhavan Road, Nr. Mann Party Plot, Thaltej, Ahmedabad – 380059
- Registration: Scan the QR code shared by HOF Pharma or email recruitment@hofpharma.com
Available Positions & Responsibilities
1. QC Officer/Sr. Officer
- Perform HPLC analysis and routine quality control checks.
- Maintain QC records and documentation.
2. Production Officer/Sr. Officer
- Manage tablet, capsule, and liquid production documentation.
- Ensure compliance with GMP guidelines.
3. ADL Sr. Officer/Executive
- Conduct analytical development using HPLC.
- Prepare and review ADL reports and documentation.
4. QA Officer/Sr. Officer
- Review and maintain quality documentation.
- Ensure compliance with SOPs and GMP.
5. DQA Officer/Sr. Officer
- Handle ADL and QA document management.
- Ensure proper record-keeping and approvals.
6. Production Documentation Cell Officer/Sr. Officer
- Prepare and review production documents.
- Maintain accurate records for GMP compliance.
Qualifications & Experience
- Education: BPharm / MPharm / MSc / BSc (relevant discipline)
- Experience:
- QC & ADL – 3-6 years in HPLC
- Production – 3-6 years in tablets, capsules, or liquid formulations
- QA & DQA – 2-4 years in documentation
- Production Documentation – 1-3 years
Benefits
- Competitive salary (INR 4–8 LPA, depending on experience)
- Exposure to WHO-GMP certified manufacturing processes
- Opportunity for career growth in formulation and quality assurance
- Immediate joining opportunities
How to Apply
- Bring hard copies of your resume, Aadhar card, and last 3 salary slips.
- Scan the QR code for registration.
- Email: recruitment@hofpharma.com