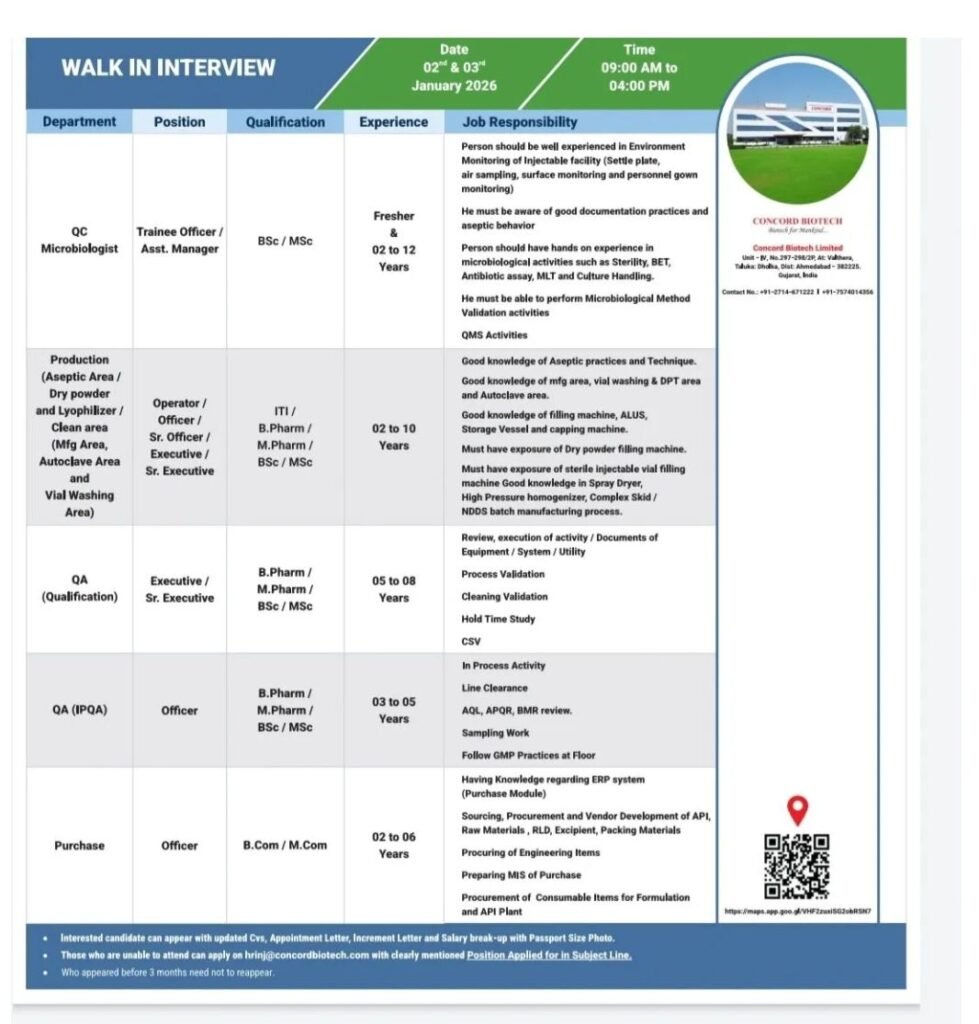
Concord Biotech Limited, a leading injectable and biotechnology pharmaceutical company, is conducting a mega walk-in interview in January 2026 for multiple positions across Quality Control, Quality Assurance, Production, and Purchase departments. This hiring drive is open to freshers as well as experienced professionals (up to 12 years), offering excellent career growth in sterile injectable manufacturing and quality operations.
Candidates with experience in aseptic practices, microbiology, injectable manufacturing, validation, and GMP documentation are highly encouraged to attend.
Walk-In Interview Details
- Interview Dates: 02 & 03 January 2026
- Time: 09:00 AM to 04:00 PM
- Mode: Walk-In Interview
- Company: Concord Biotech Limited
Open Positions & Departments
🔬 Quality Control – Microbiology
Positions: Trainee Officer / Assistant Manager
Qualification: B.Sc / M.Sc (Microbiology / Life Sciences)
Experience: Fresher to 12 Years
🏭 Production (Injectable & Sterile Areas)
Areas:
- Aseptic Area
- Dry Powder & Lyophilizer
- Clean Area (Manufacturing, Autoclave, Vial Washing)
Positions: Operator, Officer, Sr. Officer, Executive, Sr. Executive
Qualification: ITI / B.Pharm / M.Pharm / B.Sc / M.Sc
Experience: As per role
✅ Quality Assurance – Qualification
Positions: Executive / Sr. Executive
Qualification: B.Pharm / M.Pharm / B.Sc / M.Sc
Experience: 5 to 8 Years
🧪 Quality Assurance – IPQA
Position: Officer
Qualification: B.Pharm / M.Pharm / B.Sc / M.Sc
Experience: 3 to 5 Years
🛒 Purchase Department
Position: Officer
Qualification: B.Com / M.Com
Experience: 2 to 6 Years
Key Job Responsibilities
QC Microbiology
- Environmental monitoring of injectable facilities (settle plates, air sampling, surface & personnel monitoring)
- Sterility testing, BET, antibiotic assay, MLT, culture handling
- Microbiological method validation
- QMS activities and GMP documentation
- Strong aseptic behavior and data integrity compliance
Production (Sterile Injectables)
- Operation of vial washing, autoclave, filling & capping machines
- Exposure to dry powder filling and sterile vial filling machines
- Knowledge of ALUS, storage vessels, spray dryer, homogenizer
- NDDS / complex skid batch manufacturing
- Strict adherence to GMP on shop floor
Quality Assurance (IPQA & Qualification)
- Equipment, system & utility qualification
- Process validation, cleaning validation, hold time studies
- CSV activities
- Line clearance, AQL, APQR/APOR, BMR review
- In-process checks and sampling
Purchase
- ERP (Purchase module) handling
- Sourcing & procurement of APL raw materials, excipients, PM
- Vendor development & MIS preparation
- Procurement for API & formulation plants
Salary & Benefits
- Salary Range: ₹3,00,000 – ₹10,00,000 per annum (based on role & experience)
- Industry-standard benefits
- Exposure to regulated injectable manufacturing
- Long-term career growth with a reputed biotech company
Application Instructions
Walk-In Candidates
Bring the following documents:
- Updated CV
- Appointment letter
- Increment letter
- Salary breakup
- Passport-size photograph
Unable to Attend Walk-In?
Email your CV to:
📧 hrinj@concordbiotech.com