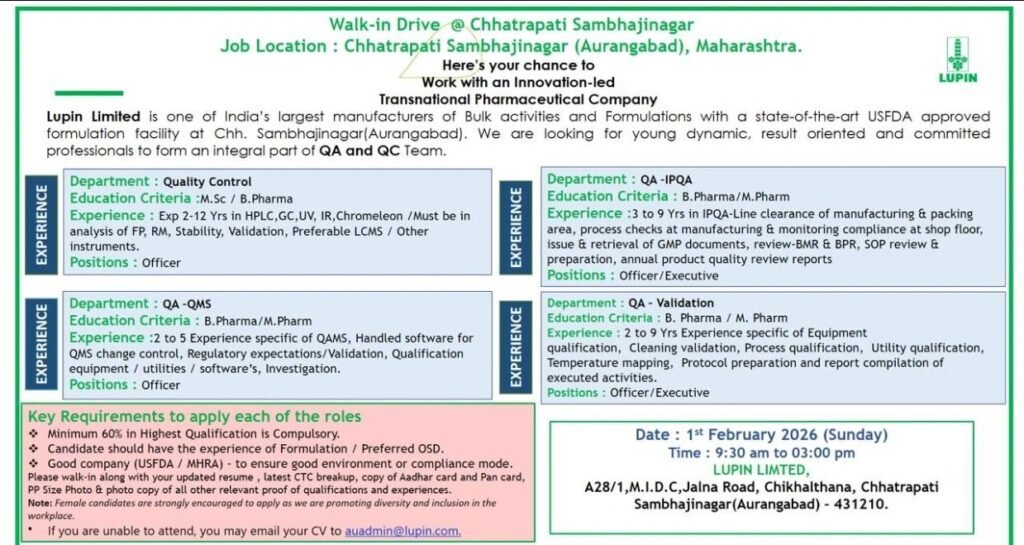
Lupin Limited, a globally recognized innovation-led transnational pharmaceutical company, is conducting a walk-in drive at its USFDA-approved formulation facility in Chhatrapati Sambhajinagar (Aurangabad), Maharashtra. This is an excellent opportunity for Quality Assurance (QA) and Quality Control (QC) professionals with experience in formulations (OSD preferred) to join a reputed compliance-driven organization.
This Lupin walk-in drive 2026 targets skilled professionals looking for long-term career growth in a regulated pharma environment aligned with USFDA and MHRA standards.
🏭 About Lupin Limited
Lupin Limited is one of India’s largest pharmaceutical manufacturers with a strong presence in bulk drugs and formulations. The company is known for its quality-first culture, regulatory excellence, and inclusive workplace practices.
📌 Job Location
Chhatrapati Sambhajinagar (Aurangabad), Maharashtra
MIDC Chikhalthana – USFDA-approved Formulation Facility
🔬 Open Positions & Job Details
1️⃣ Quality Control (QC) – Officer
Education: M.Sc / B.Pharm
Experience: 2–12 years
Key Responsibilities:
- Analysis of Finished Products (FP), Raw Materials (RM), Stability samples
- Method validation and documentation
- Operation of HPLC, GC, UV, IR
- Handling Chromeleon software
- Exposure to LC-MS preferred
2️⃣ Quality Assurance – QMS (QA-QMS) – Officer
Education: B.Pharm / M.Pharm
Experience: 2–5 years
Key Responsibilities:
- QMS change control & deviation management
- Handling QMS software
- Regulatory compliance and audit readiness
- Equipment, utility & software qualification
- Investigation and CAPA management
3️⃣ Quality Assurance – IPQA (QA-IPQA) – Officer / Executive
Education: B.Pharm / M.Pharm
Experience: 3–9 years
Key Responsibilities:
- Line clearance (manufacturing & packing)
- In-process checks and shop-floor GMP compliance
- Issuance and retrieval of GMP documents
- Review of BMR & BPR
- SOP preparation and review
- Annual Product Quality Review (APQR)
4️⃣ Quality Assurance – Validation (QA-Validation) – Officer / Executive
Education: B.Pharm / M.Pharm
Experience: 2–9 years
Key Responsibilities:
- Equipment, cleaning & process qualification
- Utility qualification & temperature mapping
- Validation protocol preparation
- Execution, review, and report compilation
✅ Key Eligibility Criteria
- Minimum 60% marks in highest qualification (mandatory)
- Experience in Formulation / OSD preferred
- Background from USFDA / MHRA regulated plants
- Strong understanding of GMP & regulatory expectations
Female candidates are strongly encouraged to apply as part of Lupin’s diversity and inclusion initiative.
💰 Salary & Benefits
- Salary Range: ₹4,00,000 – ₹12,00,000 per annum (CTC)
- Performance-based growth opportunities
- Exposure to global regulatory standards
- Inclusive, compliance-driven work culture
🗓 Walk-in Interview Details
📅 Date: 1st February 2026 (Sunday)
⏰ Time: 9:30 AM – 3:00 PM
📍 Venue:
Lupin Limited
A-28/1, MIDC, Jalna Road
Chikhalthana, Chhatrapati Sambhajinagar (Aurangabad) – 431210
📄 Documents to Carry
- Updated Resume
- Latest CTC breakup
- Aadhaar Card & PAN Card (copies)
- Passport-size photographs
- Educational & experience certificates
📧 Unable to Attend?
Email your updated CV to:
auadmin@lupin.com