Alembic Pharmaceuticals Limited, a Great Place to Work® Certified organization with over 100 years of legacy, is conducting a Walk-In Drive at Vapi, Gujarat for Formulation (OSD) and API manufacturing roles. This hiring drive targets ITI, Diploma, B.Pharm, M.Pharm, B.Sc, M.Sc, and Chemical Engineering graduates with 1–5 years of experience in regulated pharmaceutical plants.
This is an excellent opportunity for pharma professionals seeking stable careers in OSD Production, Packing, and API Manufacturing at Alembic’s Vadodara–Vapi region sites.
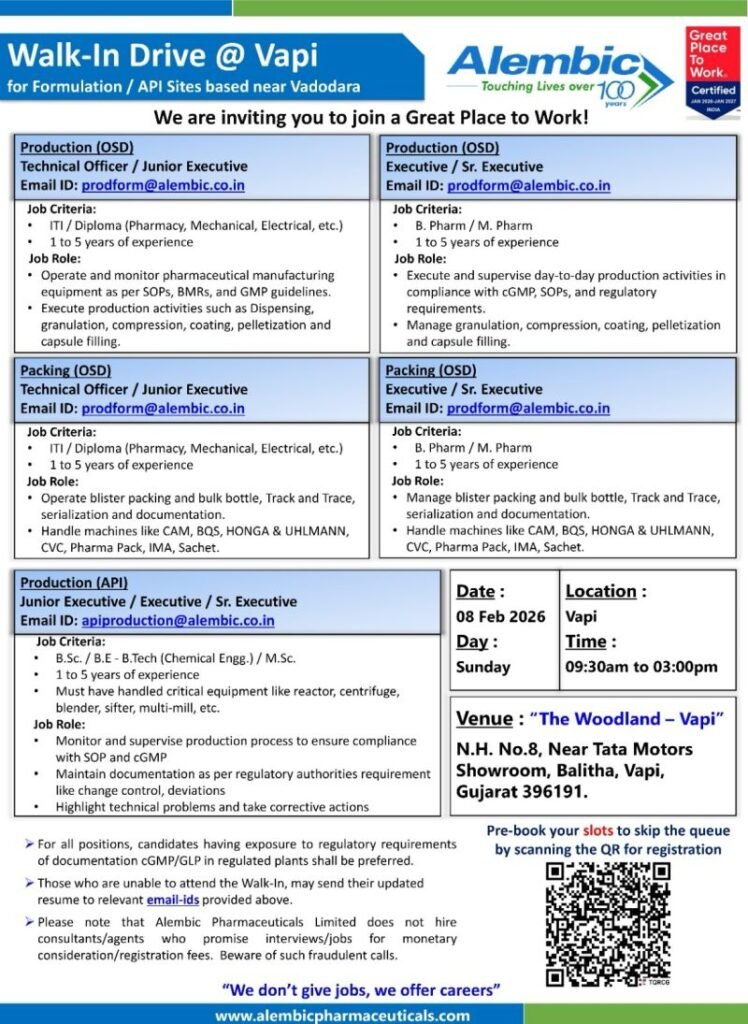
📅 Walk-In Drive Details
- Company: Alembic Pharmaceuticals Limited
- Date: 08 February 2026 (Sunday)
- Time: 09:30 AM – 03:00 PM
- Location: Vapi, Gujarat
- Venue: The Woodland – Vapi,
N.H. No. 8, Near Tata Motors Showroom, Balitha, Vapi – 396191
📌 Open Positions & Job Roles
1️⃣ Production (OSD) – Technical Officer / Junior Executive
Qualification: ITI / Diploma (Pharmacy, Mechanical, Electrical)
Experience: 1–5 years
Key Responsibilities:
- Operate pharmaceutical manufacturing equipment as per SOPs, BMRs, and cGMP
- Execute dispensing, granulation, compression, coating, pelletization, capsule filling
- Ensure line clearance and documentation compliance
2️⃣ Packing (OSD) – Technical Officer / Junior Executive
Qualification: ITI / Diploma (Pharmacy, Mechanical, Electrical)
Experience: 1–5 years
Key Responsibilities:
- Handle blister packing, bulk bottle packing, serialization, track & trace
- Operate machines like CAM, BQS, UHLMANN, HONGA, IMA, CVC, Pharma Pack
- Maintain packing documentation as per regulatory norms
3️⃣ Production (OSD) – Executive / Senior Executive
Qualification: B.Pharm / M.Pharm
Experience: 1–5 years
Key Responsibilities:
- Supervise day-to-day OSD production activities
- Ensure compliance with cGMP, SOPs, and regulatory requirements
- Manage granulation, compression, coating, pelletization & capsule filling
4️⃣ Packing (OSD) – Executive / Senior Executive
Qualification: B.Pharm / M.Pharm
Experience: 1–5 years
Key Responsibilities:
- Manage packing operations including serialization & documentation
- Troubleshoot packing line issues
- Ensure regulatory and audit readiness
5️⃣ Production (API) – Jr. Executive / Executive / Sr. Executive
Qualification: B.Sc / M.Sc / B.E / B.Tech (Chemical Engineering)
Experience: 1–5 years
Key Responsibilities:
- Operate and monitor reactors, centrifuges, blenders, sifters, multimill
- Handle deviations, change control, and technical problem-solving
- Maintain documentation as per cGMP and regulatory authority guidelines
✅ Preferred Candidate Profile
- Experience in regulated pharma manufacturing plants
- Strong knowledge of cGMP / GLP documentation
- Exposure to audits and regulatory compliance
💼 Salary & Benefits
- Estimated Salary Range: ₹2.8 LPA – ₹6.5 LPA (as per role & experience)
- Stable career with a reputed Indian pharma leader
- Learning exposure to regulated global markets
- Safe, compliant, and growth-oriented work culture
📩 How to Apply
Attend Walk-In Interview
Carry updated resume, ID proof, photographs, and relevant documents.
Unable to Attend? Email Your Resume
- OSD Production & Packing: 📧 prodform@alembic.co.in
- API Production: 📧 apiproduction@alembic.co.in