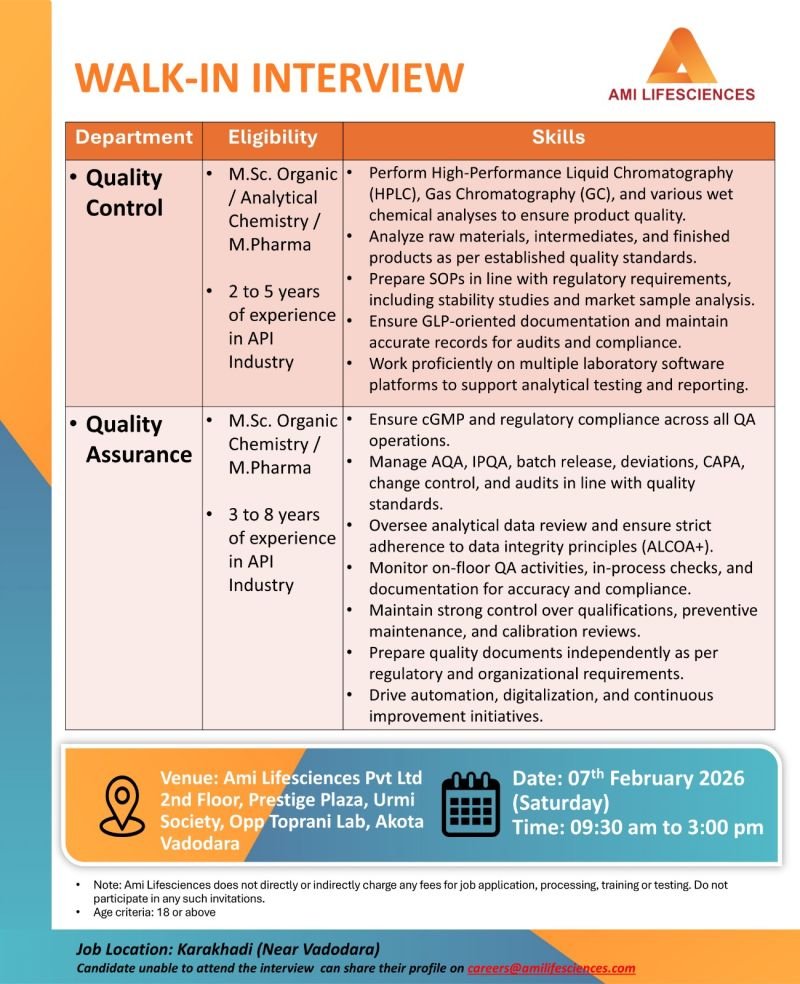
AMI Lifesciences Pvt. Ltd., a leading API pharmaceutical manufacturer, is conducting a walk-in interview in Vadodara for experienced professionals in Quality Control (QC) and Quality Assurance (QA) departments. This opportunity is ideal for M.Sc or M.Pharma candidates with hands-on experience in the API industry who are looking to advance their careers in a cGMP-compliant, regulatory-driven environment.
If you are skilled in HPLC, GC, ALCOA+, data integrity, audits, and batch release, this walk-in drive offers a strong platform for professional growth at a reputed pharma organization.
📌 Walk-In Interview Details
- Company: AMI Lifesciences Pvt. Ltd.
- Interview Type: Walk-In Interview
- Date: 07 February 2026 (Saturday)
- Time: 09:30 AM – 03:00 PM
- Venue:
Ami Lifesciences Pvt. Ltd.,
2nd Floor, Prestige Plaza,
Urmi Society, Opp. Toprani Lab,
Akota, Vadodara – Gujarat - Job Location: Karakhadi (Near Vadodara)
- Age Criteria: 18 years and above
🧪 Open Positions & Responsibilities
🔬 Quality Control (QC)
Key Responsibilities:
- Perform HPLC, GC, and wet chemical analyses for APIs
- Analyze raw materials, intermediates, and finished products
- Prepare and follow SOPs, stability studies, and market sample analysis
- Maintain GLP-compliant documentation for audits and inspections
- Work on laboratory software systems for analytical reporting
Experience Required: 2–5 years (API Industry)
🛡️ Quality Assurance (QA)
Key Responsibilities:
- Ensure cGMP and regulatory compliance across QA operations
- Handle AQA, IPQA, batch release, deviations, CAPA, change control
- Review analytical data with strict adherence to ALCOA+ data integrity
- Monitor on-floor QA activities and in-process checks
- Oversee qualification, calibration, and preventive maintenance reviews
- Drive digitalization, automation, and continuous improvement initiatives
Experience Required: 3–8 years (API Industry)
🎓 Eligibility Criteria
- Qualification:
- M.Sc. (Organic Chemistry / Analytical Chemistry)
- M.Pharma
- Industry Experience: API / Bulk Drug Manufacturing
- Skills Required:
- HPLC, GC, wet chemistry
- ALCOA+ & data integrity
- cGMP, regulatory audits
- Documentation & compliance
💼 Salary & Benefits
- Estimated Salary Range:
💰 ₹4,00,000 – ₹8,50,000 per annum (INR, based on role & experience) - Competitive compensation aligned with industry standards
- Exposure to regulatory audits and global quality systems
- Career growth in a reputed API manufacturing organization
📧 How to Apply
- Attend the walk-in interview directly at the venue with updated CV
- Unable to attend?
Email your resume to: careers@amilifesciences.com