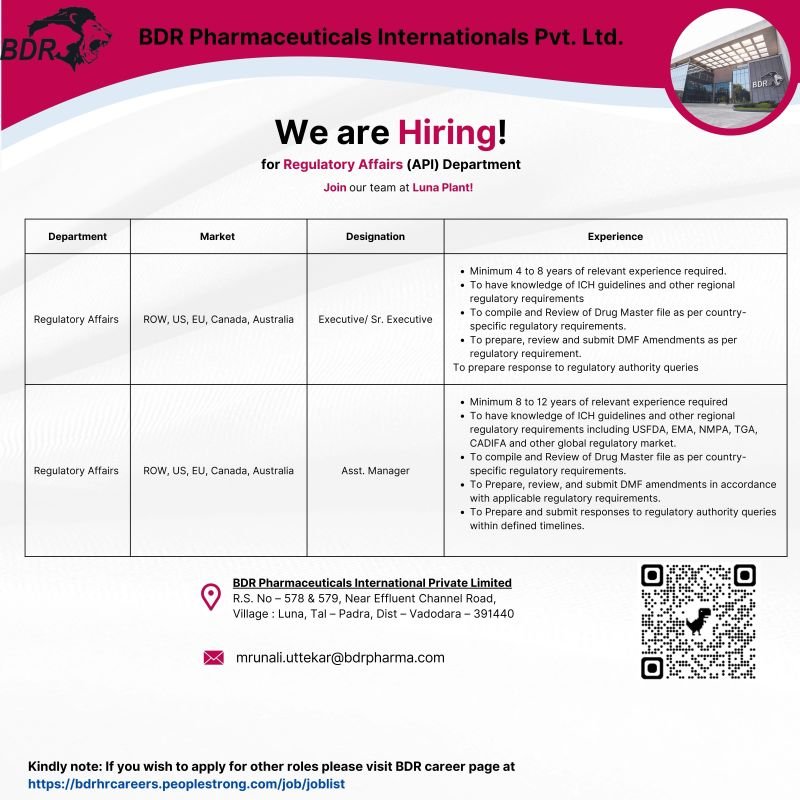
BDR Pharmaceuticals International Pvt. Ltd., a leading Indian pharmaceutical company with a strong global footprint, is inviting applications for Regulatory Affairs (API) professionals at its Luna Plant, Vadodara.
This is an excellent opportunity for experienced Regulatory Affairs professionals seeking growth in US, EU, Canada, Australia, and ROW regulatory markets, working on Drug Master Files (DMF), amendments, and regulatory authority responses.
If you are looking for Regulatory Affairs API jobs in Vadodara, BDR Pharma regulatory vacancies, or DMF regulatory affairs jobs, this opening is ideal for you.
📌 Open Positions
🔹 Regulatory Affairs Executive / Sr. Executive – API
Experience: 4 to 8 Years
🔹 Regulatory Affairs Assistant Manager – API
Experience: 8 to 12 Years
Department: Regulatory Affairs – API
Markets: ROW, US, EU, Canada, Australia
Location: Luna Plant, Vadodara (Gujarat)
🧾 Key Responsibilities
- Compile and review Drug Master Files (DMF) as per country-specific regulatory requirements
- Prepare, review, and submit DMF Amendments
- Prepare and submit responses to regulatory authority queries within defined timelines
- Ensure compliance with ICH guidelines and global regulatory requirements
- Coordinate with internal departments for dossier compilation
- Maintain regulatory documentation and submission records
🎓 Qualifications & Skills
- Bachelor’s or Master’s degree in Pharmacy / Pharmaceutical Sciences / Chemistry
- Strong knowledge of ICH guidelines and regional regulations
- Hands-on experience with USFDA, EMA, NMPA, TGA, CADIFA and other global markets
- Experience in API Regulatory Affairs and DMF lifecycle management
- Good communication and documentation skills
💰 Salary & Benefits
- Competitive salary: ₹6,00,000 – ₹14,00,000 per annum (based on role & experience)
- Career growth in a reputed pharma organization
- Professional work environment
- Learning and development opportunities
📩 How to Apply
Interested candidates can send their updated resume to:
Email: mrunali.uttekar@bdrpharma.com
🏭 Company Address
BDR Pharmaceuticals International Private Limited
R.S. No-578 & 579, Near Effluent Channel Road,
Village: Luna, Tal-Padra, Dist – Vadodara – 391440, Gujarat