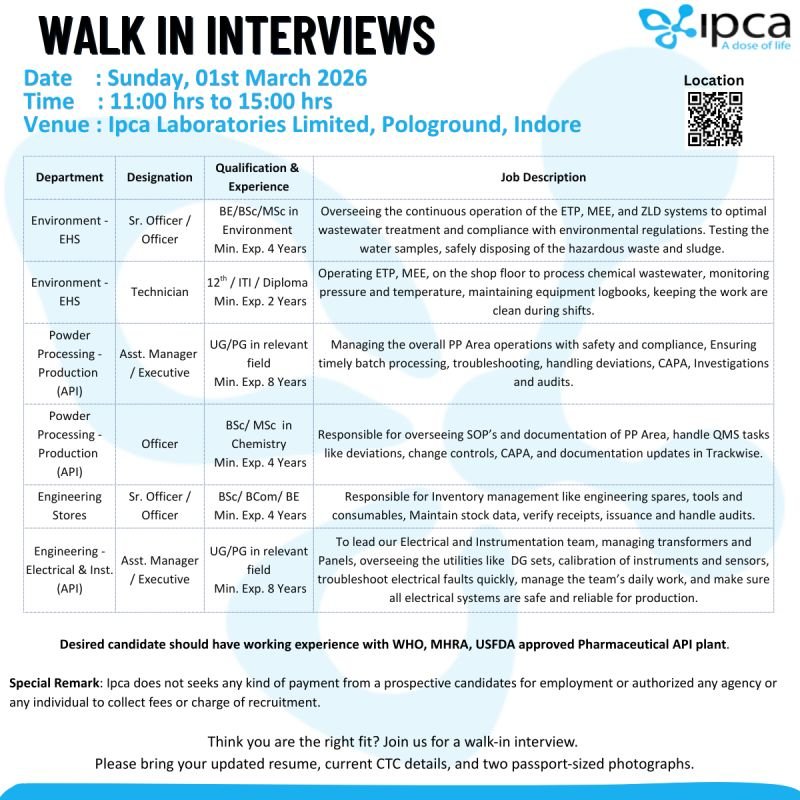
Ipca Laboratories Limited has announced a Walk-In Interview in Indore for multiple positions across API Production, Environment – EHS, Engineering Stores, and Electrical & Instrumentation departments.
This Ipca Walk-In Interview 2026 is scheduled for Sunday, 01 March 2026, at the Pologround, Indore facility, a WHO, MHRA, and USFDA-approved pharmaceutical API manufacturing plant.
If you have experience in API manufacturing, ETP/MEE/ZLD operations, electrical maintenance, or pharmaceutical QMS systems, this is a strong career opportunity in a regulated pharma environment.
🔎 Open Positions & Eligibility
1️⃣ Environment – EHS (Sr. Officer / Officer)
Qualification: BE / BSc / MSc (Environment)
Experience: Minimum 4 Years
Key Responsibilities:
- Oversee ETP, MEE, and ZLD operations
- Ensure wastewater treatment compliance
- Environmental sample testing
- Hazardous waste & sludge disposal
- Regulatory adherence
2️⃣ Environment – EHS (Technician)
Qualification: 12th / ITI / Diploma
Experience: Minimum 2 Years
Responsibilities:
- Operate ETP and MEE systems
- Monitor pressure & temperature
- Maintain equipment logbooks
- Shop floor compliance and cleanliness
3️⃣ Powder Processing – Production (API)
Asst. Manager / Executive
- UG/PG in relevant field
- Minimum 8 Years experience
Officer
- BSc/MSc (Chemistry)
- Minimum 4 Years experience
Key Responsibilities:
- Manage API powder processing operations
- Ensure batch compliance & timely processing
- Handle deviations, CAPA, audits
- QMS documentation (Trackwise)
4️⃣ Engineering Stores (Sr. Officer / Officer)
Qualification: BSc / BCom / BE
Experience: Minimum 4 Years
Responsibilities:
- Inventory management of engineering spares
- Maintain stock records
- Audit compliance
- Receipts & issuance tracking
5️⃣ Engineering – Electrical & Instrumentation (API)
Asst. Manager / Executive
- UG/PG in relevant field
- Minimum 8 Years experience
Key Responsibilities:
- Manage transformers & panels
- DG sets & utilities management
- Calibration of instruments
- Electrical fault troubleshooting
- Team leadership & compliance
🎯 Desired Candidate Profile
Candidates with experience in:
- WHO-approved API plant
- MHRA-regulated facility
- USFDA-compliant pharmaceutical manufacturing
- GMP & QMS systems
- Audit exposure
💼 Why Attend Ipca Walk-In Interview?
- Work in a regulated API manufacturing plant
- Exposure to USFDA & MHRA audits
- Growth in EHS, API Production, Engineering
- Competitive salary package
- Stable pharmaceutical organization
💰 Expected Salary (Indicative)
- Officer Level: ₹3.5 – ₹6 LPA
- Executive Level: ₹6 – ₹9 LPA
- Assistant Manager: ₹9 – ₹14 LPA
(Salary varies based on experience and regulatory exposure)
📄 Documents to Carry
- Updated Resume
- Current CTC details
- Two passport-size photographs
- Experience certificates
⚠️ Important: Ipca does not charge any recruitment fees.
📌 How to Apply?
Eligible candidates can directly attend the Ipca Laboratories Walk-In Interview in Indore on 01 March 2026 at the mentioned venue.
📅 Walk-In Interview Details
- Date: Sunday, 01 March 2026
- Time: 11:00 AM – 3:00 PM
- Venue: Ipca Laboratories Limited, Pologround, Indore
- Industry: Pharmaceutical API Manufacturing