Company: Apothecon Pharmaceuticals Pvt. Ltd.
Location: Vadodara (Dabhasa), Gujarat
Are you a skilled pharmaceutical professional looking to accelerate your career? Apothecon Pharmaceuticals Pvt. Ltd., a forward-thinking company engaged in API and Formulation manufacturing, is conducting a massive recruitment drive for its Vadodara facility. With a state-of-the-art R&D center and a commitment to quality, this is your chance to join a dynamic team.
We have multiple vacancies across our OSD Manufacturing and Quality Control departments. If you have a background in pharmacy or science and relevant experience in the pharma industry, we encourage you to apply.
Detailed Job Openings at Apothecon Pharmaceuticals
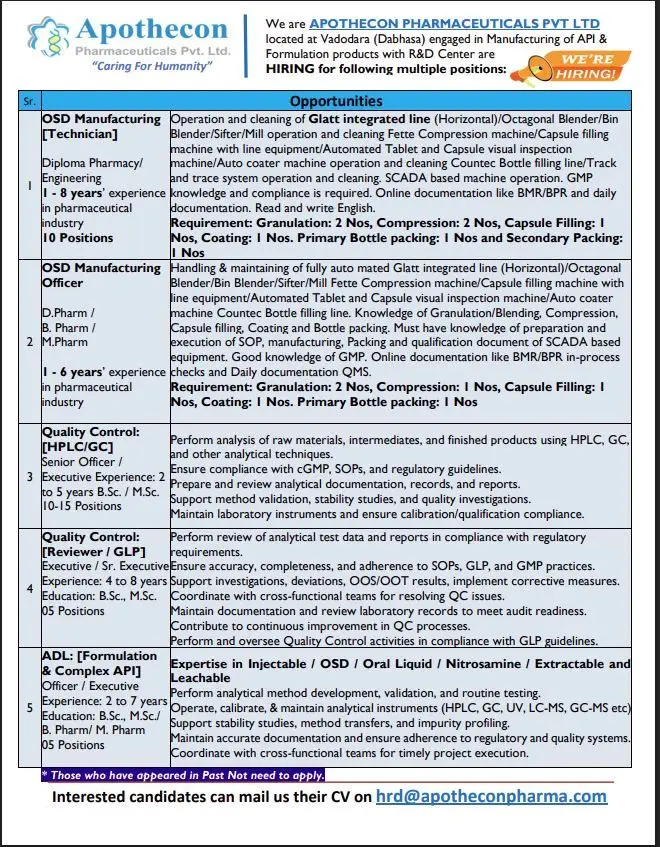
1. OSD Manufacturing – Technician
- Number of Positions: 10
- Education Required: Diploma in Pharmacy or Engineering
- Experience Required: 1 to 8 years in the pharmaceutical industry.
Key Responsibilities (Job Description):
- Operation and cleaning of key process equipment: Glatt integrated line (Horizontal), Octagonal Blender, Bin Blender, Sifter, and Mill.
- Operating and cleaning Fette Compression machines and Capsule filling machines with line equipment.
- Handling Automated Tablet & Capsule visual inspection machines and Auto coater machines.
- Operating and cleaning Countec Bottle filling lines and Track & Trace systems.
- Working with SCADA-based machine operations and maintaining strict GMP compliance.
- Handling online documentation like BMR/BPR and daily activity reports.
- Must be able to read and write in English.
Specific Departmental Requirements:
- Granulation: 2 Positions
- Compression: 2 Positions
- Capsule Filling: 1 Position
- Coating: 1 Position
- Primary Bottle Packing: 1 Position
- Secondary Packing: 1 Position
2. Officer – OSD Manufacturing
- Number of Positions: 5
- Education Required: D.Pharm / B.Pharm / M.Pharm
- Experience Required: 1 to 6 years in the pharmaceutical industry.
Key Responsibilities (Job Description):
- Handling & maintaining fully automated equipment like the Glatt integrated line, Fette Compression machine, Capsule filler, and Auto coater.
- Must have in-depth knowledge of processes like Granulation/Blending, Compression, Capsule filling, Coating, and Bottle packing.
- Preparation and execution of SOPs, manufacturing, packing, and qualification documents for SCADA-based equipment.
- Strong knowledge of Good Manufacturing Practices (GMP).
- Managing online documentation including BMR/BPR, in-process checks, and daily documentation related to QMS (Quality Management System).
Specific Departmental Requirements:
- Granulation: 2 Positions
- Compression: 1 Position
- Capsule Filling: 1 Position
- Coating: 1 Position
- Primary Bottle Packing: 1 Position
3. Quality Control – Senior Officer/Executive (HPLC/GC)
- Number of Positions: 10-15
- Education Required: B.Sc. / M.Sc.
- Experience Required: 2 to 5 years.
Key Responsibilities (Job Description):
- Perform analysis of raw materials, intermediates, and finished products using HPLC, GC, and other analytical techniques.
- Ensure compliance with cGMP, SOPs, and regulatory guidelines.
- Prepare and review analytical documentation, records, and reports.
- Support method validation, stability studies, and quality investigations.
- Maintain laboratory instruments and ensure calibration/qualification compliance.
4. Quality Control – Executive/Sr. Executive (Reviewer/GLP)
- Number of Positions: 5
- Education Required: B.Sc. / M.Sc.
- Experience Required: 4 to 8 years.
Key Responsibilities (Job Description):
- Perform review of analytical test data and reports, ensuring compliance with GLP (Good Laboratory Practice) and GMP.
- Ensure accuracy, completeness, and adherence to SOPs.
- Support investigations, deviations, OOS/OOT results, and implement corrective measures.
- Maintain documentation and review laboratory records to ensure audit readiness.
- Contribute to continuous improvement in QC processes.
5. ADL – Officer/Executive (Formulation & Complex API)
- Number of Positions: 5
- Education Required: B.Sc., M.Sc./ B.Pharm/ M.Pharm
- Experience Required: 2 to 7 years.
Key Responsibilities (Job Description):
- Expertise in Injectable / OSD / Oral Liquid / Nitrosamine / Extractable and Leachable analysis.
- Perform analytical method development, validation, and routine testing.
- Operate, calibrate, and maintain advanced instruments like HPLC, GC, UV, LC-MS, GC-MS.
- Support stability studies, method transfers, and impurity profiling.
- Maintain accurate documentation and ensure adherence to regulatory standards.
- Coordinate with cross-functional teams for timely project execution.
About Apothecon Pharmaceuticals Pvt. Ltd.
Apothecon Pharmaceuticals Pvt. Ltd., with its motto “Caring For Humanity,” is a reputable name in the pharmaceutical manufacturing sector. Located in Vadodara (Dabhasa), the company is actively engaged in the manufacturing of API (Active Pharmaceutical Ingredients) and Formulations, backed by a dedicated R&D Center. They are committed to maintaining the highest standards of quality and compliance, offering a robust platform for professionals to grow and contribute to meaningful healthcare solutions.
How to Apply
Interested and eligible candidates are requested to mail their updated CV to hrd@apotheconpharma.com.