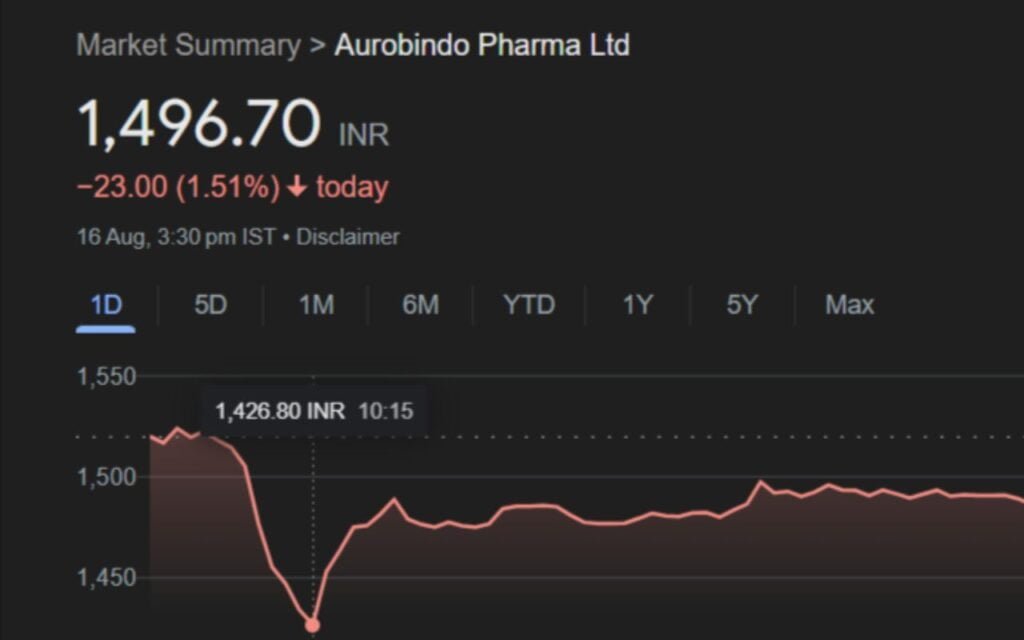
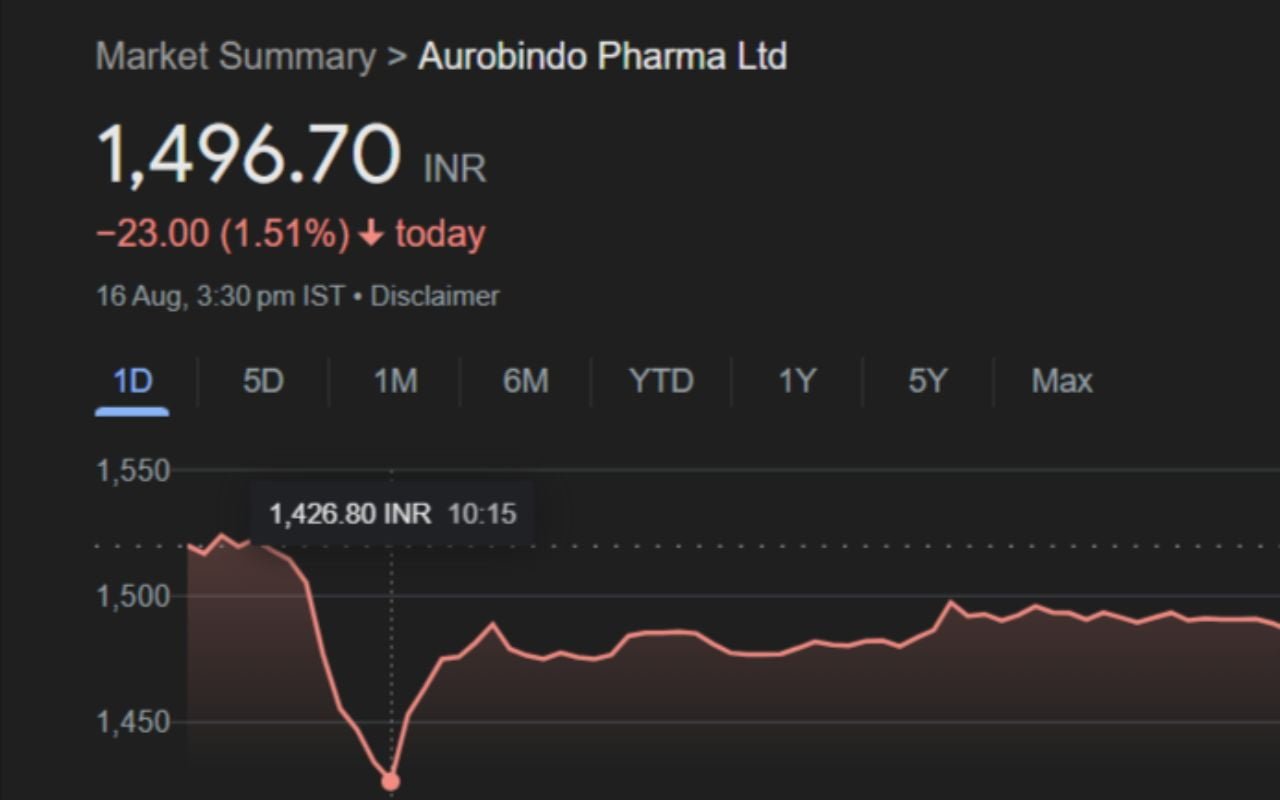
Aurobindo Pharma Limited’s shares fell on Friday, snapping a three-day winning streak following the report that the US Food and Drug Administration (USFDA) had issued a warning notice to its subsidiary’s manufacturing facility.
On Friday, Aurobindo Pharma shares fell to an intraday low of Rs 1,426.80 a share on the National Stock Exchange.
The pharmaceutical company stated in a stock exchange statement that the USFDA had issued a warning letter to Unit-III of Eugia Pharma Specialties Limited, a wholly owned subsidiary of Aurobindo Pharma. This warning followed an Official Action Indicated (OAI) status issued earlier in May, which indicated compliance concerns during an inspection.
On Friday, Aurobindo Pharma’s stock opened marginally higher on the NSE at Rs 1,525 per share, but then fell more than 6% to an intraday low of Rs 1,426.80. Despite recovering some losses, the stock closed at Rs 1,496.7, down 1.51% from its previous close.
Aurobindo Pharma has restated its commitment to addressing the US FDA’s concerns and improving compliance at its Telangana manufacturing plant. The USFDA inspection, which took place between January 22 and February 2, underlined the need for adjustments at the unit.
Despite the setback, the company remains hopeful about Eugia’s financial performance, forecasting sales of $600 million (about Rs 497 crore) in FY25. The US market is a major revenue source for Aurobindo Pharma, accounting for 47% of total sales. In the June quarter, the company’s US sales totaled $426 million (Rs 3,500 crore), down slightly from $432 million (Rs 3,626 crore) in the March quarter.
Under USFDA regulations, an OAI status may result in the withholding of approvals for any ongoing product applications or supplements relating to the facility until compliance issues are remedied.
Comments are closed.