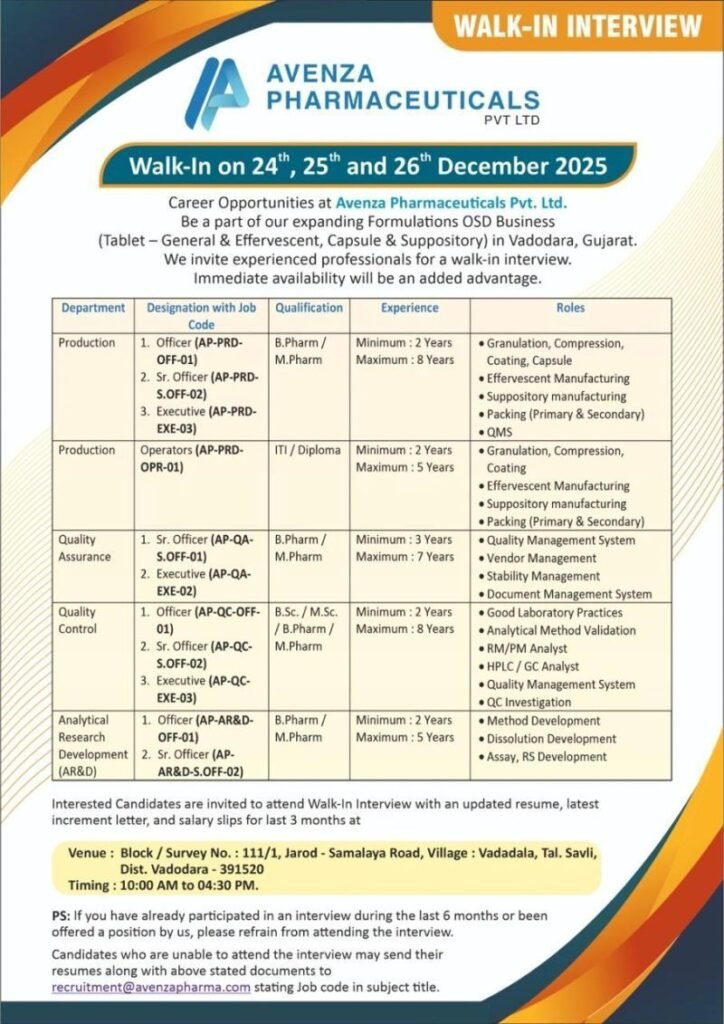
Avenza Pharmaceuticals Pvt. Ltd. is conducting a walk-in interview on 24th, 25th, and 26th December 2025 for its expanding Formulations OSD Business at Vadodara, Gujarat. This recruitment drive is ideal for experienced pharmaceutical professionals seeking opportunities in Production, Quality Assurance, Quality Control, QMS, Packing, and AR&D across Tablet (General & Effervescent), Capsule, and Suppository manufacturing.
Immediate joiners will be given preference.
Available Departments & Job Roles
1. Production – Effervescent & OSD Manufacturing
Designations & Job Codes
- Officer – AP-PRD-OFF-01
- Senior Officer – AP-PRD-S.OFF-02
- Executive – AP-PRD-EXE-03
Key Responsibilities
- Granulation, compression, coating, and capsule operations
- Effervescent and suppository manufacturing
- Primary & secondary packing operations
- Compliance with GMP and production documentation
Qualification & Experience
- B.Pharm / M.Pharm
- 2 to 8 years of relevant experience
2. Production Operators
Job Code: AP-PRD-OPR-01
Responsibilities
- Operating granulation, compression, and coating machines
- Supporting effervescent and suppository production
- Packing and shop-floor compliance
Qualification & Experience
- ITI / Diploma
- Up to 5 years of experience
3. Quality Assurance / QMS
Designations
- Senior Officer – AP-QA-S.OFF-01
- Executive – AP-QA-EXE-02
Key Responsibilities
- Vendor qualification and audits
- Stability management
- Document Management System (DMS)
- QMS compliance and regulatory documentation
Qualification & Experience
- B.Pharm / M.Pharm / B.Sc / M.Sc
- 2 to 7 years of experience
4. Quality Control (QC)
Designations
- Officer – AP-QC-OFF-01
- Senior Officer – AP-QC-S.OFF-02
- Executive – AP-QC-EXE-03
Responsibilities
- RM/PM analysis
- HPLC & GC operations
- GLP compliance and QC investigations
- Analytical method validation
Qualification & Experience
- B.Pharm / M.Pharm / B.Sc / M.Sc
- 2 to 8 years of experience
5. Analytical Research & Development (AR&D)
Designations
- Officer – AP-AR&D-OFF-01
- Senior Officer – AP-AR&D-S.OFF-02
Responsibilities
- Method development & validation
- Dissolution, assay, and related substances development
Qualification & Experience
- B.Pharm / M.Pharm
- 2 to 5 years of experience
Why Join Avenza Pharmaceuticals?
- Growing formulations company with strong OSD portfolio
- Exposure to effervescent and suppository technologies
- Structured quality systems and compliance culture
- Competitive salary aligned with pharma industry standards
- Career growth in production, QA, QC, and AR&D domains
Walk-In Interview Details
Company: Avenza Pharmaceuticals Pvt. Ltd.
Dates: 24th, 25th & 26th December 2025
Time: 10:00 AM to 04:30 PM
Venue:
Block/Survey No. 111/1,
Jarod – Samalaya Road,
Village Vadadala, Tal. Savli,
Dist. Vadodara – 391520, Gujarat
Documents to Carry
- Updated resume
- Latest increment letter
- Salary slips (last 3 months)
Note: Candidates interviewed or offered a position in the last 6 months should not attend.
Unable to Attend the Walk-In?
Send your resume and supporting documents to:
📧 recruitment@avenzapharma.com