Centaur Pharmaceuticals Pvt Ltd is a leading specialty drug manufacturing organization based in Pune, India. Renowned for producing high-quality, affordable medicines, Centaur is trusted by healthcare professionals worldwide and complies with stringent regulatory standards such as USFDA and MHRA. With a commitment to innovation and excellence, Centaur is rapidly expanding its operations and seeking talented professionals to join its dynamic team.
Why Work at Centaur Pharmaceuticals?
- Industry Leader: Largest specialty drug manufacturer in India.
- Global Compliance: Products approved by USFDA, MHRA, and other global agencies.
- Growth Opportunities: Rapidly expanding company with career advancement potential.
- Innovative Environment: Work with cutting-edge pharmaceutical technologies.
- Competitive Salary & Benefits: Attractive remuneration packages for qualified candidates.
Current Job Openings at Centaur Pharmaceuticals, Pune
Centaur Pharmaceuticals is hiring for multiple positions in formulation, oral solid dosage, quality control, production, and maintenance departments. Below are the details:
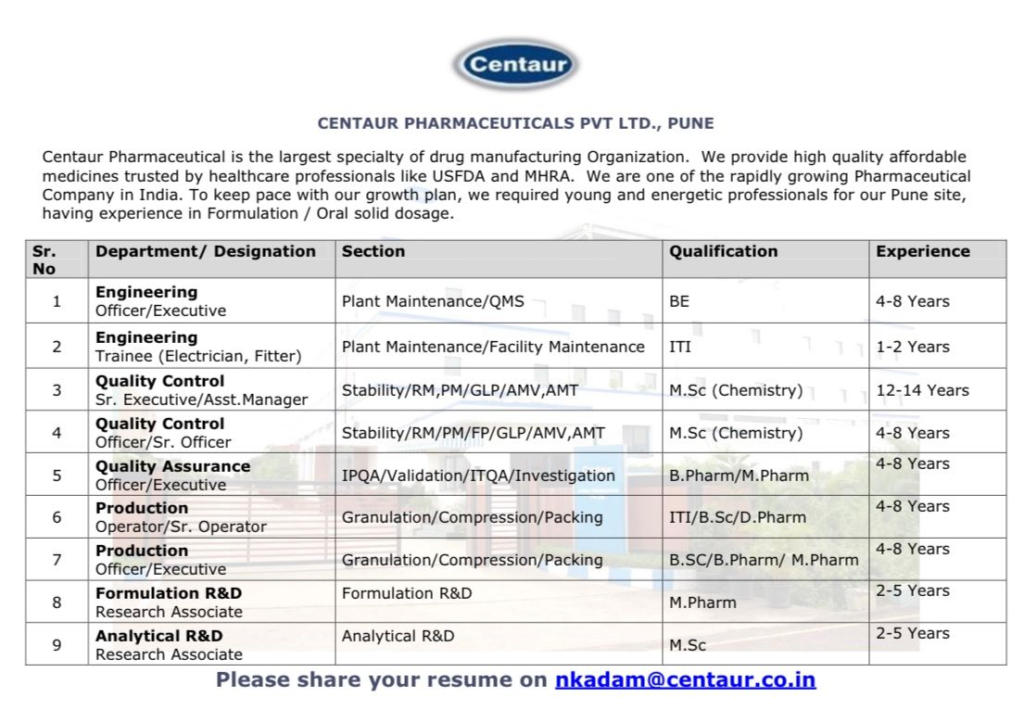
| Sr. No | Department / Designation | Section | Qualification | Experience |
|---|---|---|---|---|
| 1 | Engineering Officer / Executive | Plant Maintenance / QMS | BE | 4-8 Years |
| 2 | Engineering Trainee (Electrician, Fitter) | Plant Maintenance / Facility Maintenance | ITI | 1-2 Years |
| 3 | Quality Control Sr. Executive / Asst. Manager | Stability / RM, PM / GLP / AMV, AMT | M.Sc (Chemistry) | 12-14 Years |
| 4 | Quality Control Officer / Sr. Officer | Stability / RM / PM / FP / GLP / AMV, AMT | M.Sc (Chemistry) | 4-8 Years |
| 5 | Quality Assurance Officer / Executive | IPQA / Validation / ITQA / Investigation | B.Pharm / M.Pharm | 4-8 Years |
| 6 | Production Operator / Sr. Operator | Granulation / Compression / Packing | ITI / B.Sc / D.Pharm | 4-8 Years |
| 7 | Production Officer / Executive | Granulation / Compression / Packing | B.Sc / B.Pharm / M.Pharm | 4-8 Years |
| 8 | Formulation R&D Research Associate | Formulation R&D | M.Pharm | 2-5 Years |
| 9 | Analytical R&D Research Associate | Analytical R&D | M.Sc | 2-5 Years |
Job Descriptions
Engineering Officer / Executive (Plant Maintenance / QMS)
- Oversee plant maintenance activities ensuring compliance with quality management systems.
- Manage preventive maintenance and troubleshoot equipment issues.
- Coordinate with production and quality teams to maintain operational efficiency.
Engineering Trainee (Electrician, Fitter)
- Assist in facility maintenance and electrical repairs.
- Support senior engineers in daily maintenance tasks.
- Ensure safety and compliance with plant standards.
Quality Control Sr. Executive / Assistant Manager
- Lead stability studies and raw material testing.
- Ensure compliance with GLP and regulatory guidelines.
- Manage quality control documentation and reporting.
Quality Control Officer / Sr. Officer
- Conduct routine quality checks on raw materials, in-process, and finished products.
- Maintain laboratory instruments and ensure data integrity.
- Support quality assurance initiatives.
Quality Assurance Officer / Executive
- Perform in-process quality assurance and validation activities.
- Investigate deviations and implement corrective actions.
- Ensure compliance with regulatory audits and inspections.
Production Operator / Sr. Operator
- Operate granulation, compression, and packing machinery.
- Maintain production records and adhere to SOPs.
- Ensure product quality and safety standards.
Production Officer / Executive
- Supervise production processes and team members.
- Coordinate with quality and maintenance departments.
- Ensure timely production targets and compliance.
Formulation R&D Research Associate
- Develop and optimize oral solid dosage formulations.
- Conduct stability and compatibility studies.
- Document research findings and support scale-up activities.
Analytical R&D Research Associate
- Perform analytical method development and validation.
- Conduct routine analysis of raw materials and finished products.
- Maintain laboratory compliance and documentation.
How to Apply
Interested candidates with the required qualifications and experience are invited to send their updated resume to:
Email: nkadam@centaur.co.in