Dr. Reddy’s Laboratories Ltd. is an integrated pharmaceutical company committed to providing affordable and innovative medicines. With a robust presence across the globe, Dr. Reddy’s is known for its excellence in Active Pharmaceutical Ingredient (API) manufacturing, product development, and a strong quality ethos. Working here means being part of a culture that values scientific excellence, integrity, and patient-centricity.
Job Openings & Eligibility
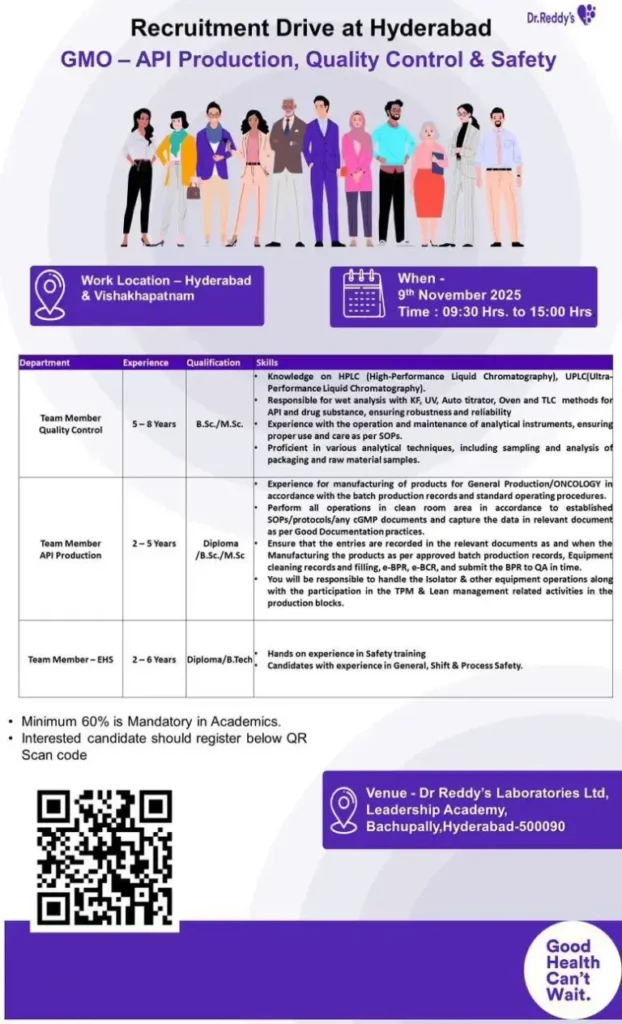
The walk-in interview is scheduled for 9th November 2025. Here are the detailed vacancies:
| Department | Role | Experience Required | Qualification Required |
|---|---|---|---|
| Quality Control | Team Member | 5 – 8 Years | B.Sc. / M.Sc. |
| API Production | Team Member | 2 – 5 Years | Diploma / B.Sc. / M.Sc. |
| EHS (Safety) | Team Member | 2 – 6 Years | Diploma / B.Tech |
Note: A minimum of 60% in academics is mandatory for all positions.
Detailed Job Description
1. Team Member – Quality Control
As a Quality Control Team Member, you will be at the forefront of ensuring the quality and efficacy of pharmaceutical products.
- Key Skills & Responsibilities:
- In-depth knowledge of HPLC (High-Performance Liquid Chromatography) and UPLC (Ultra-Performance Liquid Chromatography).
- Perform wet analysis using KF (Karl Fischer), UV Spectrophotometer, Auto Titrator, Oven, and TLC (Thin-Layer Chromatography) methods for API and drug substances.
- Operate, maintain, and calibrate a wide range of analytical instruments as per Standard Operating Procedures (SOPs).
- Proficient in sampling and analysis of raw materials and packaging samples.
- Ensure all data is recorded with robustness and reliability, adhering to cGMP guidelines.
2. Team Member – API Production
Join the API Production team and play a vital role in manufacturing life-saving medicines.
- Key Skills & Responsibilities:
- Experience in manufacturing products for General Production or ONCOLOGY as per batch production records (BPR) and SOPs.
- Perform all operations within a clean room area, strictly following cGMP and Good Documentation Practices (GDP).
- Accurately record data in electronic Batch Production Records (e-BPR), Batch Cleaning Records (BCR), and ensure timely submission to the QA department.
- Operate specialized equipment like Isolators.
- Actively participate in TPM (Total Productive Maintenance) and Lean Management initiatives to enhance productivity.
3. Team Member – EHS (Environment, Health & Safety)
Ensure a safe and compliant working environment as part of the EHS team.
- Key Skills & Responsibilities:
- Hands-on experience in conducting Safety Training for employees.
- Candidates with a background in General Safety, Shift Safety, and Process Safety are preferred.
- Implement and monitor safety protocols to maintain a zero-harm workplace.
How to Apply for Dr. Reddy’s Recruitment Drive
This is a walk-in interview. Interested and eligible candidates can directly attend the drive at the venue below.
- Date: 9th November 2025
- Time: 09:30 AM to 3:00 PM
- Venue: Dr. Reddy’s Laboratories Ltd, Leadership Academy, Bachupally, Hyderabad – 500090.