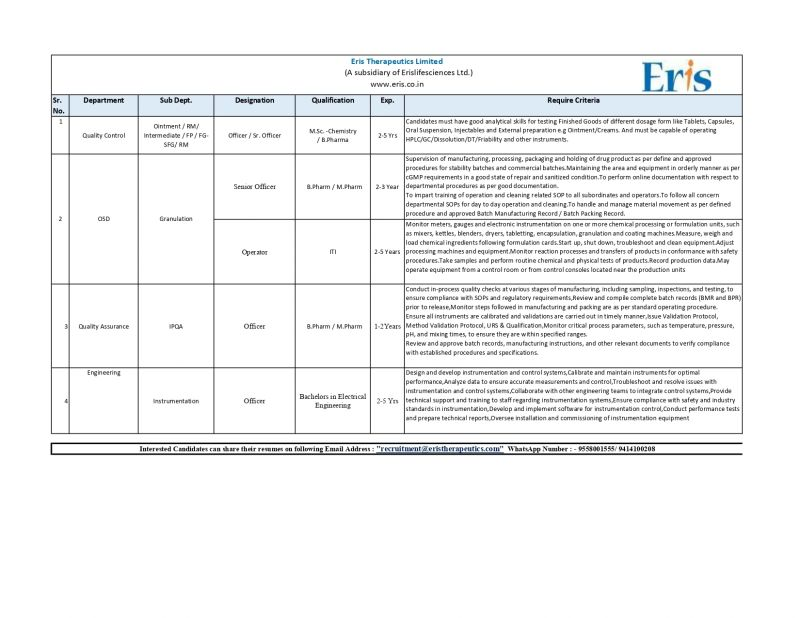
Eris Therapeutics Limited (a subsidiary of Eris Lifesciences Ltd) has announced multiple openings across Quality Control, Quality Assurance, OSD Production, Granulation and Engineering-Instrumentation departments. These pharma jobs in Ahmedabad are ideal for candidates with hands-on experience in OSD, manufacturing, analytical instruments, and GMP-compliant operations.
If you’re looking for pharma production jobs, QA jobs, QC jobs, or pharma engineering jobs, this is a great opportunity to join a reputed and fast-growing organization.
Available Positions & Responsibilities
1. Quality Control – Officer / Sr. Officer
Qualification: M.Sc Chemistry / B.Pharm
Experience: 2–5 years
Department: Ointment, RM, Intermediate, FP/FG
Key Responsibilities:
- Perform analytical testing of Tablets, Capsules, Oral Suspensions, Injectables, and External Preparations.
- Operate HPLC, GC, Dissolution, DT, Friability testers, and other instruments.
- Ensure compliance with GMP and SOP standards.
- Maintain accurate analytical documentation.
2. OSD Production – Officer / Sr. Officer
Experience: 2–3 years
Key Responsibilities:
- Supervise manufacturing, processing, packaging, and stability/commercial batches.
- Maintain GMP compliance and ensure equipment cleanliness & readiness.
- Execute online documentation as per GDP norms.
- Train operators on cleaning and operational SOPs.
- Handle material movement as per BMR/BPR.
- Operate mixers, blenders, kettles, granulation, encapsulation, and coating machines.
- Conduct routine in-process checks and maintain logs.
3. Granulation Operator
Experience: 2–3 years
Key Responsibilities:
- Operate granulation machinery including RMG, FBD, Blenders, Sifters.
- Load ingredients as per formulation sheets.
- Perform equipment startup/shutdown and cleaning.
- Monitor parameters and ensure batch consistency.
- Record production data and perform routine tests.
4. Quality Assurance – Officer / Sr. Officer (GA)
Qualification: B.Pharm / M.Pharm
Experience: 1–2 years
Key Responsibilities:
- Conduct in-process quality checks and sampling.
- Review BMR & BPR before batch release.
- Monitor temperature, pressure, pH, and other CPPs.
- Issue validation protocols, qualification documents, URS.
- Ensure calibration and validation activities.
- Approve manufacturing instructions & QA documents.
5. Engineering – Instrumentation Officer
Qualification: B.E Electrical Engineering
Experience: 3–5 years
Key Responsibilities:
- Design, develop, and maintain instrumentation & control systems.
- Perform instrument calibration and troubleshooting.
- Integrate control systems with production equipment.
- Conduct performance tests and prepare reports.
- Support commissioning of instrumentation equipment.
- Ensure safety & regulatory compliance.
Required Skills & Criteria
- Strong understanding of GMP, GDP, and regulatory compliance.
- Hands-on experience with pharma manufacturing or analytical equipment.
- Ability to maintain documentation and follow SOPs.
- Good teamwork, technical knowledge, and discipline.
Benefits of Working With Eris Therapeutics
- Opportunity to work with a leading Indian pharmaceutical manufacturer.
- Exposure to advanced equipment and regulated work environments.
- Competitive salary and growth-oriented workplace.
How to Apply
Interested candidates can share their updated resume via:
📧 Email: recruitment@eristherapeutics.com