Are you a passionate pharmaceutical professional looking to kickstart or advance your career in Quality Assurance (QA)? Puerto Life Sciences Pvt. Ltd., a leading name in the pharmaceutical industry, is hiring for multiple QA roles at its state-of-the-art sterile facility in Neemrana, Rajasthan. Whether you are a fresher or an experienced professional, we have opportunities tailored for you. Read on to explore the roles, responsibilities, and how you can be a part of our dynamic team.

Why Choose Puerto Life Sciences Pvt. Ltd.?
At Puerto Life Sciences, we believe in fostering a culture of excellence, innovation, and integrity. Our sterile facility in Neemrana is equipped with cutting-edge technology, ensuring the highest standards of quality and compliance. We are committed to delivering life-saving pharmaceutical products while maintaining strict adherence to Good Manufacturing Practices (GMP). Join us and be a part of a team that values your skills, encourages growth, and rewards dedication.
Current Job Openings in Quality Assurance
We are currently hiring for the following roles in the Quality Assurance department:
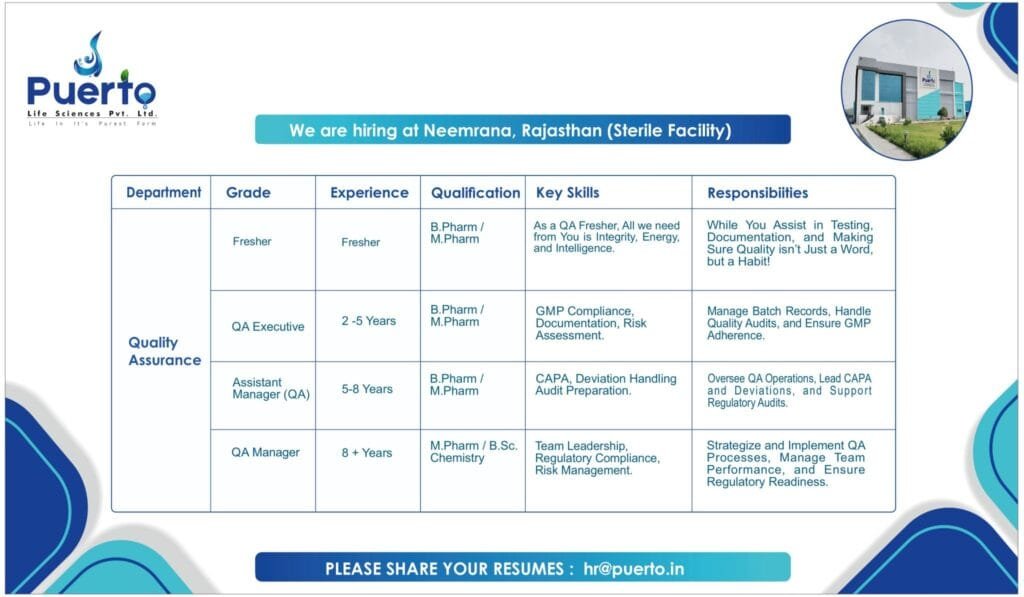
- QA Fresher
- Qualification: B.Pharm/M.Pharm
- Key Skills: Integrity, Energy, Intelligence
- Responsibilities: Assist in testing, documentation, and ensuring quality is a habit.
- QA Executive
- Experience: 2-5 years
- Qualification: B.Pharm/M.Pharm
- Key Skills: GMP Compliance, Documentation, Risk Assessment
- Responsibilities: Manage batch records, handle quality audits, and ensure GMP adherence.
- Assistant Manager (QA)
- Experience: 5-8 years
- Qualification: B.Pharm/M.Pharm
- Key Skills: CAPA, Deviation Handling, Audit Preparation
- Responsibilities: Oversee QA operations, lead CAPA and deviations, and support regulatory audits.
- QA Manager
- Experience: 8+ years
- Qualification: M.Pharm/B.Sc. Chemistry
- Key Skills: Team Leadership, Regulatory Compliance, Risk Management
- Responsibilities: Strategize and implement QA processes, manage team performance, and ensure regulatory readiness.
Key Skills and Qualifications We Look For
- Strong understanding of GMP, regulatory compliance, and quality systems.
- Proficiency in documentation, risk assessment, and audit preparation.
- Leadership skills for managerial roles to guide and mentor teams.
- Problem-solving abilities to handle deviations and implement CAPA effectively.
How to Apply
If you are ready to take the next step in your career, share your resume with us at hr@puerto.in. Make sure to mention the position you are applying for in the subject line.