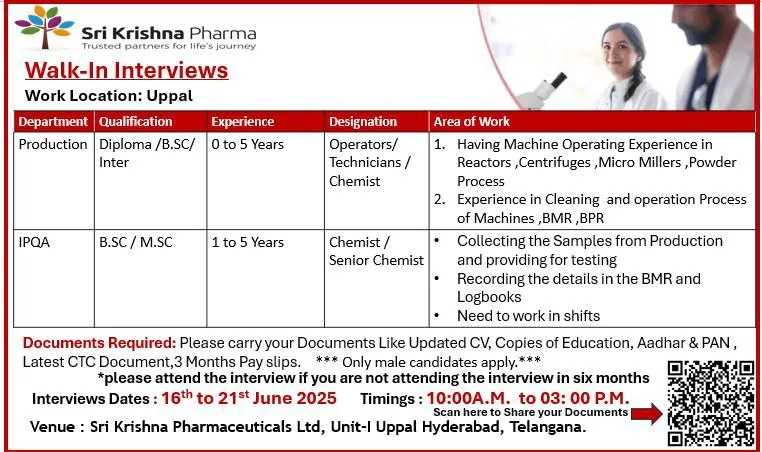
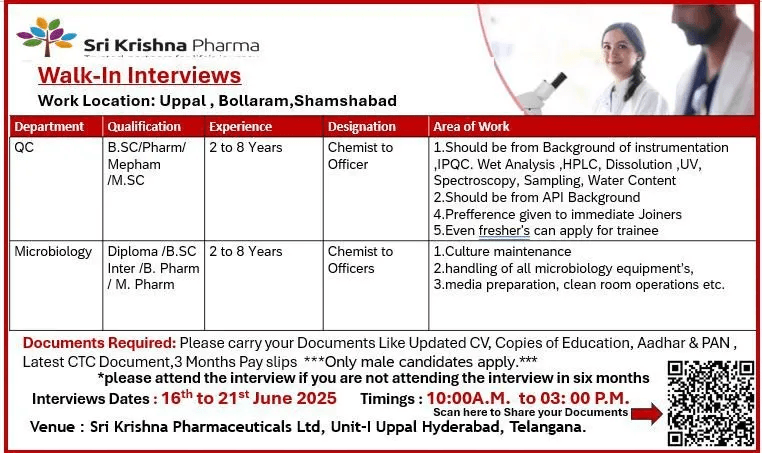
Are you looking for a career in the pharmaceutical industry? Sri Krishna Pharmaceuticals Ltd is conducting a walk-in interview for freshers and experienced professionals in Production, QC, Packing, IPQA, and Microbiology departments. If you hold qualifications like Inter, Diploma, B.Sc, M.Sc, B.Pharm, or M.Pharm, this is your chance to join a reputed pharmaceutical company in Hyderabad.
📌 Job Details
- Company: Sri Krishna Pharmaceuticals Ltd
- Location: Hyderabad (Unit-II, Nacharam)
- Departments: Production / Quality Control (QC) / Packing / IPQA / Microbiology
- Experience: 0 to 8 Years
- Qualifications: Inter, Diploma, B.Sc, M.Sc, B.Pharm, M.Pharm
- Selection Process: Face-to-face interview
- Interview Rounds: HR Round
📅 Walk-in Interview Details
- Date: 16th to 21st June 2025
- Time: 2:00 PM to 3:00 PM
- Venue:
Sri Krishna Pharmaceuticals Limited, Unit-II, Nacharam, Hyderabad
📝 Documents to Carry
- Updated Resume (CV)
- Passport-size photograph
- Copies of educational certificates
- Aadhar Card & PAN Card
- Latest CTC documents (if experienced)
- Last 3 months’ payslips & bank statement
🏢 About Sri Krishna Pharmaceuticals Ltd
Sri Krishna Pharmaceuticals Ltd (SKPL) is a leading pharmaceutical company based in Hyderabad, specializing in Active Pharmaceutical Ingredients (APIs) and finished dosages. With a strong presence in both domestic and international markets, SKPL is known for its quality manufacturing practices and innovation in pharmaceuticals.
🔍 Job Description
1. Production Department
- Handling manufacturing processes as per GMP guidelines.
- Operating and maintaining production equipment.
- Ensuring compliance with SOPs and safety protocols.
2. Quality Control (QC) Department
- Performing chemical and instrumental analysis of raw materials and finished products.
- Documentation and reporting as per regulatory standards.
- Ensuring compliance with GMP/GLP norms.
3. Packing Department
- Supervising packaging operations.
- Ensuring proper labeling and batch numbering.
- Maintaining packing line efficiency.
4. IPQA (In-Process Quality Assurance)
- Monitoring production processes for quality compliance.
- Conducting in-process checks and audits.
- Ensuring adherence to cGMP standards.
5. Microbiology Department
- Conducting microbial testing of raw materials, water, and finished products.
- Maintaining sterility testing and environmental monitoring.
- Ensuring compliance with USP/EP standards.
📌 How to Apply?
Interested candidates can directly attend the walk-in interview at the given venue between 16th to 21st June 2025 (2:00 PM – 3:00 PM).
📍 Interview Address:
Sri Krishna Pharmaceuticals Ltd, Unit-II, Nacharam, Hyderabad