Glenmark Pharmaceuticals Ltd. is a leading integrated, research-led global organization with a presence across generics, specialty, and OTC businesses. With a mission to “Enrich Lives,” Glenmark is renowned for its innovative research and development, manufacturing excellence, and a robust portfolio of products that reach patients in over 80 countries.
Working at Glenmark means being part of a culture that values innovation, quality, and integrity. Their state-of-the-art manufacturing facilities, like the one in Baddi, are designed to meet global standards, offering employees a dynamic environment to grow and excel in their pharma careers.
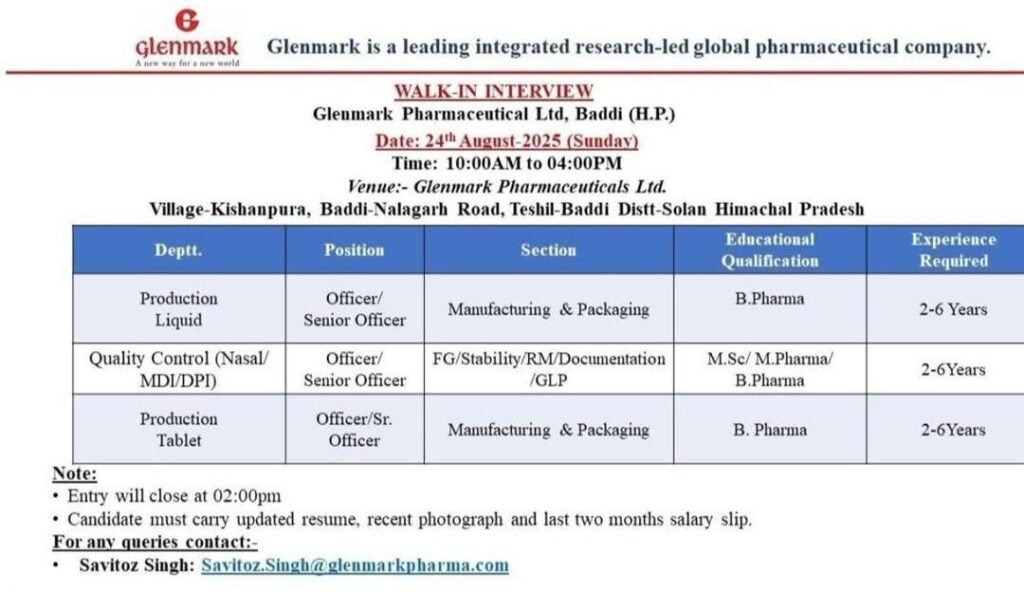
Open Positions and Job Description
Glenmark Baddi is looking for experienced candidates for its Production and Quality Control departments. Here are the detailed job openings:
1. Position: Officer / Senior Officer – Production (Liquid & Tablet)
- Department: Production
- Section: Manufacturing & Packaging (for both Liquid Oral and Tablet formulations)
- Educational Qualification: B.Pharma is mandatory.
- Experience Required: 2 to 6 years in pharmaceutical manufacturing and packaging operations.
- Job Description:
- Oversee and execute the manufacturing and packaging processes for liquid or solid dosage forms.
- Ensure adherence to Standard Operating Procedures (SOPs) and cGMP guidelines.
- Handle and operate sophisticated pharmaceutical machinery and equipment.
- Maintain batch manufacturing and packaging records accurately.
- Achieve production targets while ensuring the highest standards of quality and safety.
2. Position: Officer / Senior Officer – Quality Control (Nasal/MDI/DPI)
- Department: Quality Control
- Section: FG (Finished Goods), Stability, RM (Raw Materials), Documentation, GLP (Good Laboratory Practices).
- Educational Qualification: M.Sc / M.Pharma / B.Pharma.
- Experience Required: 2 to 6 years in a pharmaceutical QC lab. Specialized experience in Nasal sprays, Metered-Dose Inhalers (MDI), or Dry Powder Inhalers (DPI) is highly preferred.
- Job Description:
- Perform quality testing of raw materials, in-process samples, and finished goods.
- Conduct stability studies and analyze products at various interval points.
- Ensure all documentation is maintained as per GLP and regulatory requirements.
- Operate advanced analytical instruments like HPLC, GC, Dissolution apparatus, etc.
- Prepare and review quality control protocols and reports.
Walk-In Interview Details
- Date: Sunday, 24th August 2025
- Time: 10:00 AM to 04:00 PM
- Venue: Glenmark Pharmaceuticals Ltd., Village-Kishanpura, Baddi-Nalagarh Road, Tehsil-Baddi, District-Solan, Himachal Pradesh.
- Important Note: Entry will be closed sharp at 02:00 PM. Please arrive early to ensure your participation.
Mandatory Documents to Carry:
- Updated Resume: Clearly highlighting your experience in production or quality control.
- Recent Photograph: Carry a passport-sized photograph.
- Last Two Months’ Salary Slips: This is a strict requirement. Please do not forget them.
- (Optional but advisable) Copies of your educational and experience certificates.
Contact for Queries
For any specific questions regarding these pharma jobs in Baddi, you may contact:
Savitoz Singh
Email: Savitoz.Singh@glenmarkpharma.com