Lupin Limited is a global pharmaceutical company headquartered in India, renowned for its innovation, quality, and commitment to healthcare. With a strong presence in both domestic and international markets, Lupin specializes in branded and generic formulations, biotechnology products, and APIs (Active Pharmaceutical Ingredients). The company is known for its compliance with global regulatory standards including USFDA and MHRA, making it a preferred employer for pharma professionals.
Job Openings at Lupin Pithampur Indore
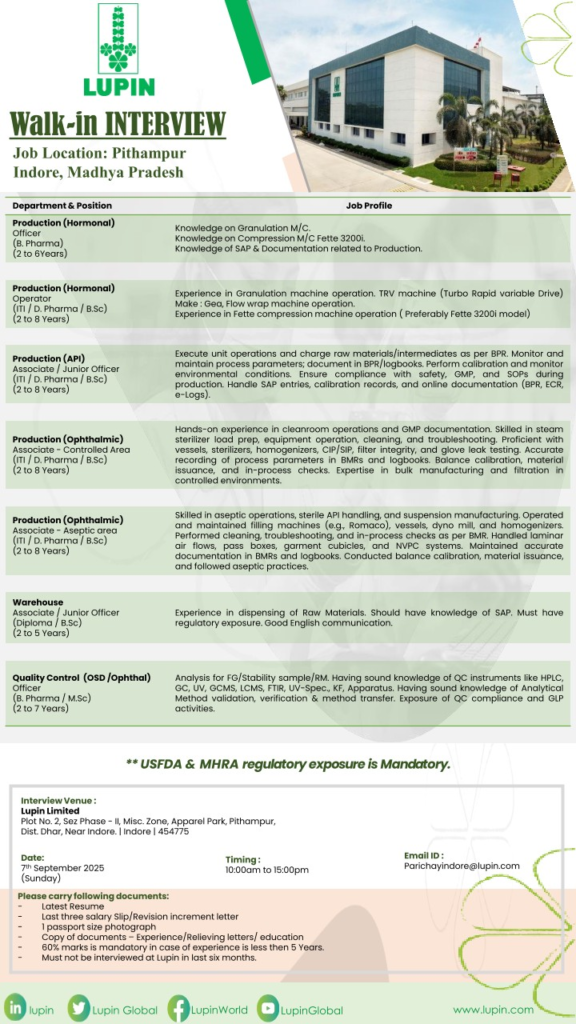
Production Department
- Production (Hormonal) Officer
Qualification: B. Pharma | Experience: 2 to 6 Years - Production (Hormonal) Operator
Qualification: ITI/D. Pharma/B.Sc | Experience: 2 to 8 Years - Production (API) Associate/Junior Officer
Qualification: ITI/D. Pharma/B.Sc | Experience: 2 to 8 Years - Production (Ophthalmic) Associate Controlled Area
Qualification: ITI/D. Pharma/B.Sc | Experience: 2 to 8 Years - Production (Ophthalmic) Associate Aseptic Area
Qualification: ITI/D. Pharma/B.Sc | Experience: 2 to 8 Years - Warehouse Associate/Junior Officer
Qualification: Diploma/B.Sc | Experience: 2 to 5 Years
Quality Control Department
- Quality Control (OSD/Ophthal) Officer
Qualification: B. Pharma/M.Sc | Experience: 2 to 7 Years
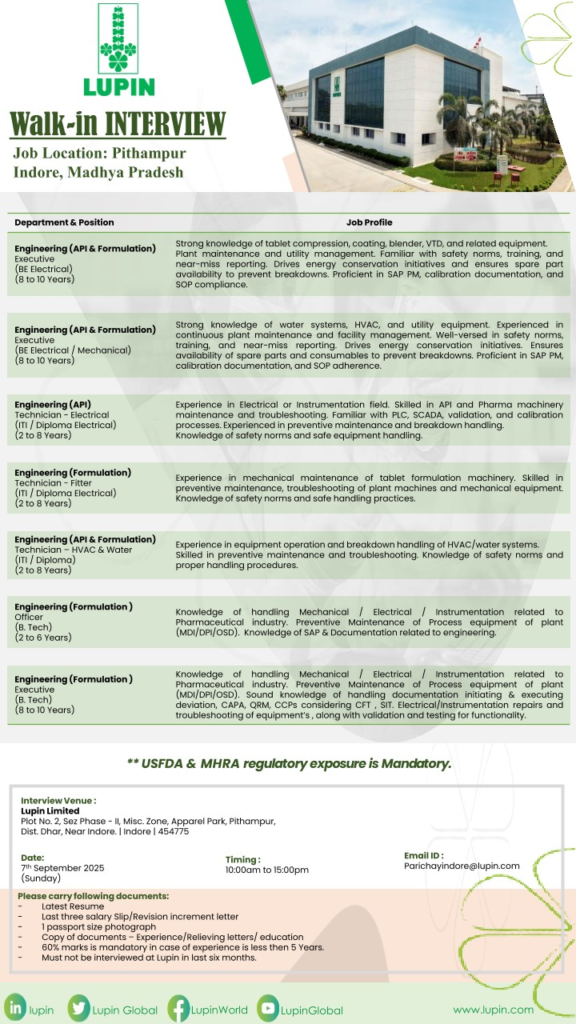
Engineering Department
- Engineering (API & Formulation) Executive
Qualification: BE Electrical/Mechanical | Experience: 8 to 10 Years - Engineering (API) Technician-Electrical
Qualification: ITI/Diploma Electrical | Experience: 2 to 8 Years - Engineering (Formulation) Technician-Fitter
Qualification: ITI/Diploma Electrical | Experience: 2 to 8 Years - Engineering (API & Formulation) Technician-HVAC & Water
Qualification: ITI/Diploma | Experience: 2 to 8 Years - Engineering (Formulation) Officer
Qualification: B.Tech | Experience: 2 to 6 Years - Engineering (Formulation) Executive
Qualification: B.Tech | Experience: 8 to 10 Years
Job Description & Key Responsibilities
Production Roles
- Operate and maintain granulation, compression (Fette 3200i), TRV (Turbo Rapid Variable Drive), and flow wrap machines.
- Execute unit operations, charge raw materials/intermediates as per Batch Production Records (BPR).
- Monitor process parameters, perform calibration, and maintain documentation (BPR, ECR, e-Logs).
- Ensure compliance with GMP, safety, and SOPs.
- Handle SAP entries and maintain calibration records.
- Work in cleanroom environments with aseptic operations, steam sterilizers, homogenizers, and filtration systems.
- Manage sterile API handling, suspension manufacturing, and filling machines (e.g., Romaco).
- Dispense raw materials and maintain regulatory compliance.
Quality Control Roles
- Conduct analysis of Finished Goods (FG), Stability samples, and Raw Materials (RM).
- Operate QC instruments such as HPLC, GC, UV, GCMS, LCMS, FTIR, KF apparatus.
- Perform analytical method validation, verification, and method transfer.
- Ensure compliance with GLP and regulatory standards including USFDA and MHRA.
Engineering Roles
- Maintain and troubleshoot tablet compression, coating, blender, VTD, HVAC, water systems, and utility equipment.
- Perform preventive maintenance and breakdown handling.
- Manage SAP PM, calibration documentation, and SOP adherence.
- Handle electrical/instrumentation repairs, PLC, SCADA, validation, and testing.
- Drive energy conservation initiatives and ensure spare part availability.
- Ensure safety compliance and conduct training on safety norms.
Eligibility Criteria
- Minimum 60% marks in educational qualifications if experience is less than 5 years.
- Relevant diploma, degree, or ITI qualifications as per the position.
- Experience as specified for each role.
- USFDA & MHRA regulatory exposure is mandatory for Quality Control and Engineering roles.
- Candidates must not have attended any Lupin interview in the last six months.
Interview Details
- Date: 7th September 2025 (Sunday)
- Time: 10:00 AM to 3:00 PM
- Venue: Lupin Limited, Plot No. 2, SEZ Phase-II, Misc. Zone, Apparel Park, Pithampur, Dist. Dhar, Near Indore, Madhya Pradesh – 454775
- Contact Email: Parichayindore@lupin.com
Documents to Carry
- Latest Resume
- Last three salary slips or revision/increment letters
- Passport size photograph (1)
- Copies of educational certificates, experience, and relieving letters
How to Apply
Interested candidates can directly attend the walk-in interview at the venue mentioned above on the specified date and time. Ensure you carry all the required documents and meet the eligibility criteria. For any queries, contact via email at Parichayindore@lupin.com.