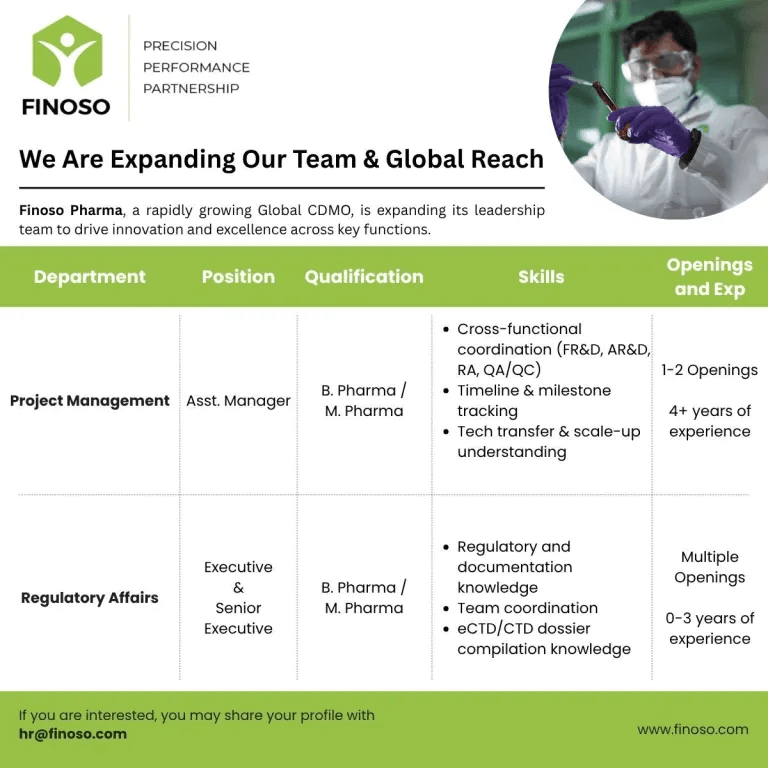
Finoso Pharma is conducting a walk-in interview for B.Pharma and M.Pharma graduates in Hyderabad for roles in Project Management & Regulatory Affairs. If you have 0–4 years of experience, this is a great opportunity to join a leading pharmaceutical company.
📌 Job Description
1. Project Management (4+ Years Experience)
- Coordinate with cross-functional teams (FR&D, AR&D, RA, QA/QC).
- Track project timelines and ensure smooth execution.
- Handle technology transfer processes and project documentation.
2. Regulatory Affairs (0–3 Years Experience)
- Prepare and review regulatory submissions (eCTD/CTD).
- Assist in dossier compilation for domestic & international markets.
- Work closely with teams to ensure compliance with regulatory guidelines.
About Finoso Pharma
Finoso Pharma is a leading pharmaceutical CDMO (Contract Development & Manufacturing Organization) known for its expertise in drug development, regulatory compliance, and project management. The company focuses on innovation and quality, making it a preferred employer in the pharma industry.
How to Apply?
🔹 Walk-In Interview Details:
- Location: Hyderabad (Exact venue shared after shortlisting)
🔹 Email Application:
- Send your resume to hr@finoso.com with the subject line:
- “Application for Project Management – Finoso Pharma” (for PM roles)
- “Application for Regulatory Affairs – Finoso Pharma” (for RA roles)
🔹 Selection Process:
- Face-to-face interview (HR Round)
- Candidates with relevant experience will be preferred.