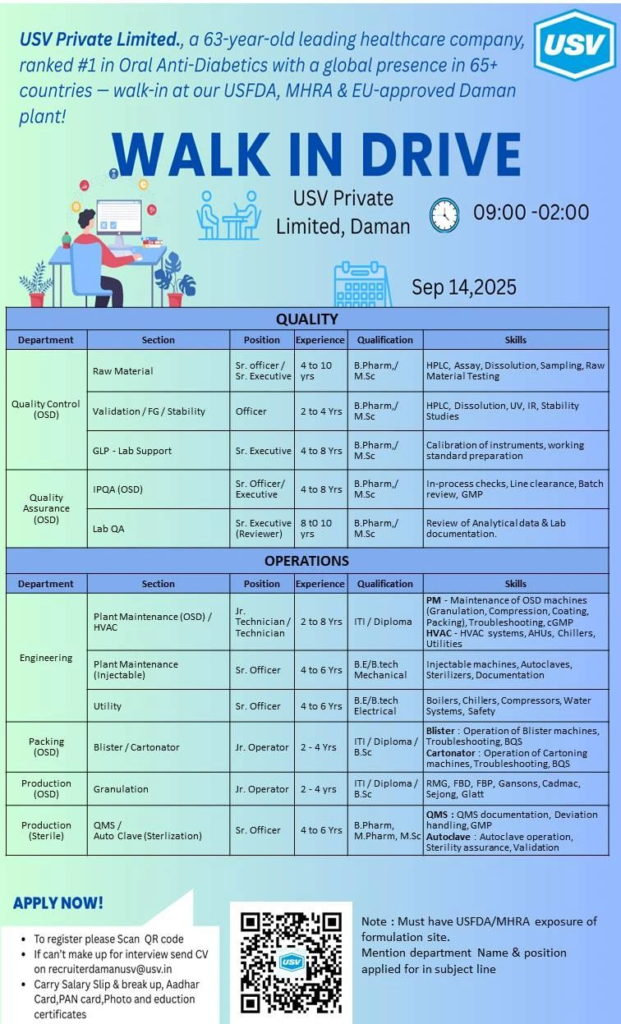
USV Private Limited is a leading pharmaceutical company known for its commitment to quality and innovation. Located in Daman, the company specializes in manufacturing oral solid dosage (OSD) and sterile injectable formulations. With a strong focus on compliance with global regulatory standards such as USFDA and MHRA, USV offers a dynamic work environment for professionals seeking growth in the pharma industry.
Job Openings & Requirements
Quality Control (Raw Material & Lab QA)
- Positions: Sr. Officer/Sr. Executive, Officer, Sr. Executive (Reviewer)
- Experience: 2 to 10 years
- Qualifications: B.Pharm, M.Sc, M.Pharm
- Skills: HPLC, Assay, Dissolution, Sampling, Raw Material Testing, UV, IR, Stability Studies, Calibration, Analytical Data Review, GLP Lab Support
Quality Assurance (IPQA & QA)
- Positions: Sr. Officer/Executive
- Experience: 4 to 8 years
- Qualifications: M.Sc, B.Pharm
- Skills: In-process checks, Line clearance, Batch review, GMP compliance
Plant Maintenance (OSD, HVAC, Injectable)
- Positions: Jr. Technician, Technician, Sr. Officer
- Experience: 2 to 8 years
- Qualifications: ITI, Diploma, BE/B.Tech Mechanical or Electrical
- Skills: Maintenance of OSD machines, HVAC systems, Autoclaves, Boilers, Chillers, Compressors, Utilities
Production (OSD & Sterile)
- Positions: Jr. Operator, Sr. Officer
- Experience: 2 to 6 years
- Qualifications: 8th Pass, ITI, Diploma, B.Pharm, M.Sc
- Skills: Granulation, Compression, Coating, Packing, Autoclave operation, Sterility assurance, Troubleshooting, CGMP compliance
Job Description
Candidates will be responsible for ensuring the highest standards of quality and compliance in their respective departments. Key responsibilities include:
- Conducting raw material testing and analytical procedures using HPLC, UV, IR, and dissolution methods.
- Performing in-process quality checks, batch reviews, and documentation in line with GMP.
- Maintaining and troubleshooting plant equipment including HVAC, autoclaves, and production machinery.
- Supporting validation, stability studies, and GLP lab operations.
- Ensuring compliance with USFDA and MHRA regulatory requirements.
How to Apply
- Walk-In Date: September 14, 2025
- Time: 9:00 AM to 2:00 PM
- Venue: USV Private Limited, Daman
- Documents to Carry: Updated CV, Salary Slip & breakup, Aadhar Card, PAN Card, Passport size photo, Educational certificates
- Email: If unable to attend, send your CV to recruiterdamanusv@usv.in
- Subject Line: Mention the department name and position applied for