Indian Drugs Recalled From USA : Defected Drugs

Indian drug manufacturers The US Food and Drug Administration (USFDA) has announced that Aurobindo Pharma, Glenmark, and FDC are recalling products in the US because of manufacturing defects. The painkiller Healthy Living Acetaminophen, Aspirin (NSAID), and Caffeine tablets are being recalled from 240 bottles by Aurobindo Pharma USA Inc., a division of the Hyderabad-based pharmaceutical ...
Lilly Zepbound drug (Weigh Loss) Is Prices in Half Now But there’s Catch
Lilly expands US supply of Zepbound by Inventing single-dose vials for weight loss. Which Will Help US Citizen Lilly Told that 86% of Insurance health plans pay for medications that treat obesity. A month’s supply of Zepbound may otherwise require patients who are not eligible for weight reduction coverage, such as those enrolled in the ...
Indian Pharma Factory Blast, 17 People Died

A catastrophic explosion at a pharmaceuticals manufacturing plant in Andhra Pradesh, southern India, has resulted in at least 17 deaths and 41 injuries. The incident occurred at Escientia Advanced Sciences, a company specializing in the production of intermediate chemicals and active pharmaceutical ingredients, located in the Achutapuram Special Economic Zone. The explosion took place at ...
Liquidia Biopharma Challenges USFDA’s Exclusivity Grant for Rival Lung Drug

Liquidia Corp has filed a lawsuit against the United States Food and medicine Administration (FDA) over its recent decision to grant United Therapeutics a three-year regulatory exclusivity for its inhaled medicine, Tyvaso DPI. The FDA’s decision, announced on Monday, significantly delays the regular clearance procedure for Liquidia’s competing inhaled medicine, Yutrepia, which is similarly intended ...
Another Countries Banned Spices Imports From India, Because Indians Eating “Pesticide”

In a remarkable development that has sparked global worries, nearly 12% of spice samples tested by Indian officials did not match the required quality and safety criteria. This finding comes after thorough inspections, sampling, and testing of mixed spice mixes in response to contamination concerns raised by two of India’s most popular spice brands, MDH ...
AstraZeneca’s blockbuster medicine got USFDA Approval, Shares Made High Record
Imfinzi, AstraZeneca’s blockbuster medicine, has received FDA permission for extended use in non-small cell lung cancer (NSCLC), marking a significant milestone in cancer treatment. With the FDA’s approval, Imfinzi can now be used as an extra post-surgery treatment for adult patients, potentially reshaping the cancer care landscape. Imfinzi, which is already a market leader in ...
AI Demand Increasing in Indian Healthcare And Pharma

The demand for artificial intelligence (AI) is fast expanding in India’s healthcare and pharmaceutical industries, as businesses see its potential to transform the industry. Artificial intelligence is being used to cut drug discovery costs, shorten time to market, and improve clinical results. In healthcare, AI helps to streamline patient care, reduce misdiagnosis, and enable individualized ...
Novartis Try To Block Generic Version of Heart Drug Entresto

Recently, Novartis, a multinational pharmaceutical corporation with headquarters in Switzerland, suffered a serious legal setback in its fight against MSN Pharmaceuticals to stop the firm from releasing a generic version of Entresto, the company’s best-selling heart failure medication. U.S. District Judge Richard Andrews in Delaware rejected Novartis’ request for a preliminary injunction, which would have ...
Mpox Vaccines Delayed, Outbreak in Central Africa
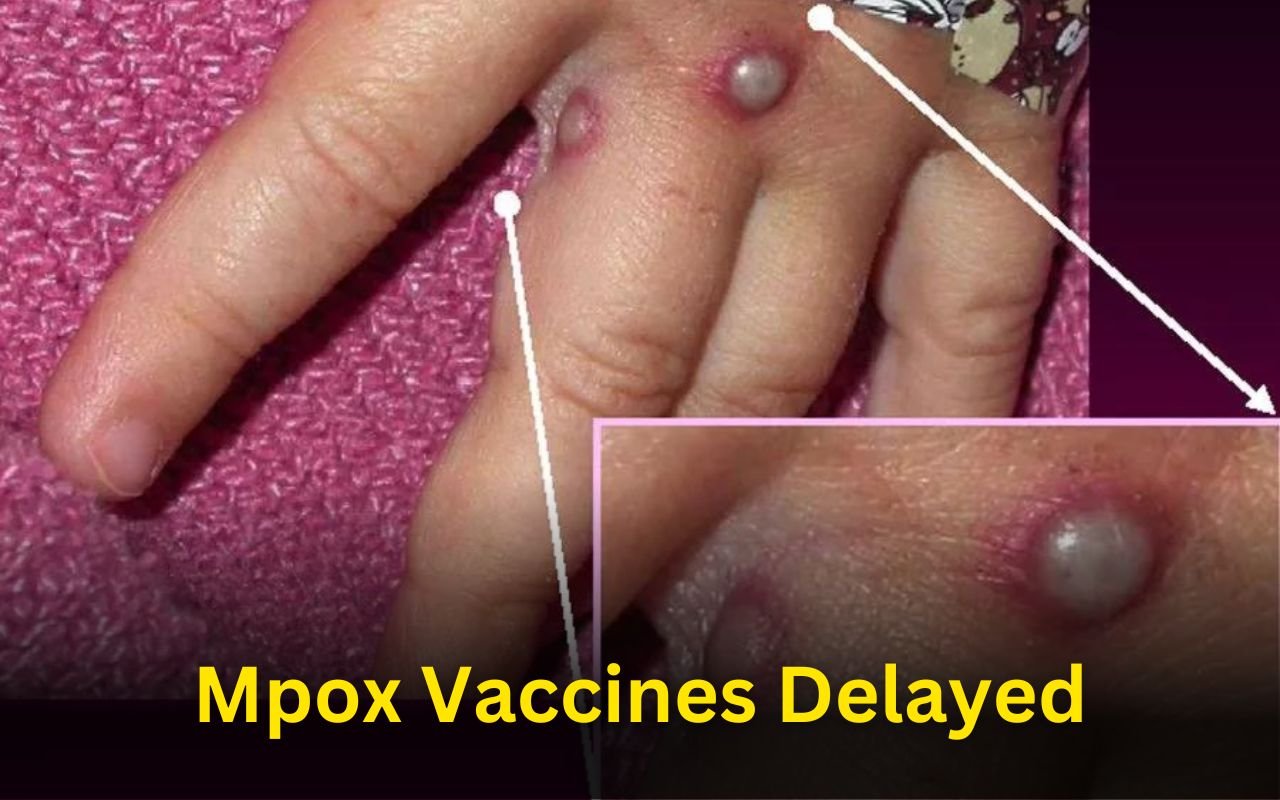
The mpox outbreak in the Democratic Republic of the Congo (DRC) and its surrounding countries is getting worse, but vaccinations to stop its spread might not be available for several months. In spite of this, the World Health Organization (WHO) and the Africa Centers for Disease Control and Prevention (Africa CDC) are still working to ...
Pfizer and BioNTech’s Combined Flu-COVID Vaccine Falls Short in Phase 3 Trial Goal

Pfizer and BioNTech recently announced that their experimental mRNA-based combination vaccine, which was supposed to protect against both influenza and COVID-19, failed not meet one of the major goals in a Phase 3 clinical trial. The findings of this late-stage experiment have led the businesses to reconsider the future of their ambitious combo vaccine development. ...
